2. 广西南海珊瑚礁研究重点实验室,广西南宁 530004
2. Guangxi Laboratory on the Study of Coral Reefs in the South China Sea, Nanning, Guangxi, 530004, China
有机紫外吸收剂(Organic Ultraviolet Absorbers,OUVs)是一类能够吸收紫外线A (Ultraviolet A,UVA,320-400 nm)和紫外线B (Ultraviolet B,UVB,280-320 nm)波段紫外线的有机化合物[1],分子结构中通常包含一个或多个苯环结构。OUVs包括有机紫外线过滤剂(Organic Ultraviolet Filter,OUVF)和有机紫外线稳定剂(Organic Ultraviolet Stabilizers,OUVS),前者主要应用于化妆品、防晒霜等个人护肤品;后者主要应用于塑料、油漆、纺织品及家具等工业产品[2]。按照化学结构划分,OUVs分为二苯甲酮类、苯并三唑类、樟脑衍生物类、对氨基苯甲酸类、肉桂酸酯类和水杨酸酯类等类型[2](图 1)。OUVs因具有较大的辛醇水分配系数(log Kow> 2),亲脂性较强,容易在生物体内累积,对生物具有内分泌干扰[3]、遗传毒性[4]、致畸性[5]和致死性[6]等。为减少OUVs的暴露风险,多个国家和地区(如欧盟[7]、日本[8]和美国夏威夷[9]等)已通过立法限制护肤品中OUVs的添加种类及最大添加量。

|
| 图 1 常见OUVs的名称和结构 Fig.1 Names and structures of common OUVs |
OUVs通过游客游泳、冲浪等水上娱乐活动,地下水径流,河流输入等多种方式进入海洋环境[10-12](图 2),在海水、沉积物和海洋生物(如海豚[13]、珊瑚[14]、贻贝[15]等)中广泛存在。为系统了解全球海洋OUVs的污染态势及其生态风险,本文总结近15年来OUVs在全球海水、沉积物和海洋生物中的赋存特征和时空分布,并结合风险商(Risk Quotient,RQ)法评估海水中OUVs对海洋生态健康的危害。

|
| 图 2 OUVs进入海洋环境的途径 Fig.2 Pathways of OUVs releasing into the marine environment |
1 材料与方法 1.1 文献调研
文献搜索条件如下。数据库网站:web of science;主题:marine;标题:ultraviolet absorbent * or UV filter * or ultraviolet filter * or sunscreen * or UV stablilizer or UV-absorbing * or UV chemical;年份:2006-2022。期刊限制在Journal of Chromatography A、Science of the Total Environment、Environmental Science & Technology、Talanta、Chenosphere、Environmental Pollution等与环境科学或分析化学相关的自然科学期刊。根据以上原则共筛选出313篇文献,然后根据文献标题和摘要进一步筛选,最终选定65篇文献。
1.2 数据分析本文采用风险商法对海水中OUVs的暴露水平进行生态风险评价。风险商指实测环境浓度(Measured Environmental Concentration,MEC)值和预测无效应浓度(Predicted No Effect Concentration,PNEC)值的比值,其中,PNEC值是指欧盟风险评价技术导则文件中推荐用于化合物环境风险评价的安全阈值[16],其数值可通过评价因子(Assessment Factor,AF)法或物种敏感度分布(Species Sensitivity Distribution,SSD)曲线法获得。当RQ≥1时,为高风险;0.1≤RQ<1时,为中风险;0.01≤RQ<0.1时,为低风险;RQ < 0.01时,几乎无风险[17]。
AF法要求从文献或数据库中获取毒物的半致死浓度(Lethal Concentration 50%,LC50)、半数有效浓度(Effective Concentration 50%,EC50)或无观察效应浓度(No Observed Effect Concentration,NOEC)值,为保守评估毒物对生态系统不同营养级生物的危害,通常选取最小LC50(或EC50、NOEC)值除以相应的AF值[18],即得到PNEC值。其中,急性毒理实验选择LC50或EC50,而慢性毒理实验选择NOEC,没有NOEC值时选择最低可见效应浓度(Lowest Observed Effect Concentration,LOEC)值的1/2作为NOEC值[19]。考虑到海洋环境较淡水环境更为复杂,海洋生物毒性的AF值选择比淡水生物高一个数量级,例如,当至少有一种来自3个营养级(鱼类、甲壳类和藻类)的急性LC50或EC50值时,AF值取10 000[20, 21]。由于本文搜集到的LC50和EC50数据均是基于急性毒理实验开展而获得的,因此OUVs的AF值均选择10 000。SSD曲线法是基于不同物种对化合物的敏感性遵循正态分布的一种计算PNEC值的方法[22]。美国国家环境保护局规定,拟合SSD曲线选用的毒理数据(LC50或EC50)需要来自3门8科,数据不少于5个(最好大于10个),且数据质量较好[23]。基于统计考虑[物种危害浓度(HCx)太小,风险预测不可靠]和环境保护需求(HCx应尽可能地小)的折中,通常选取5%物种危害浓度(HC5)值作为PNEC值[18]。
针对每个研究海域,如无特殊说明,OUVs的浓度取该海域不同采样点或生物样本的平均值。在海水、沉积物和生物样本的时空分布热图分析(Origin 2021)中,OUVs浓度经过对数转化,目的是使浓度梯度呈现更直观,对比更明显。SSD曲线拟合中,以浓度作为横坐标,累积概率(p)作为纵坐标,利用logistic拟合函数在Origin 2021中构建不同海洋生物对OUVs的SSD曲线。在生态风险评价部分,为保守评估OUVs对该海域的生态环境健康风险,如同一个研究海域有不同年份或不同季节的浓度数据,MEC取其最大值[24-26]。
2 结果与分析 2.1 OUVs在海洋环境中的赋存特征和时空分布目前已在海洋环境(海水[14, 26-42]、沉积物[11, 14, 33, 43-47]、海洋生物[10, 13-15, 33, 34, 40])中开展分析研究的OUVs种类包括二苯酮-3 (BP-3)、甲氧基肉桂酸乙基己酯(EHMC)、奥克立林(OC)、4-甲基苄亚基樟脑(4-MBC)、辛基二甲基对氨基苯甲酸(OD-PABA)、胡莫柳酯(HMS)、阿伏苯宗(AVO)等36种,研究海域主要集中在热带和亚热带旅游区或经济发达城市的周围海域。
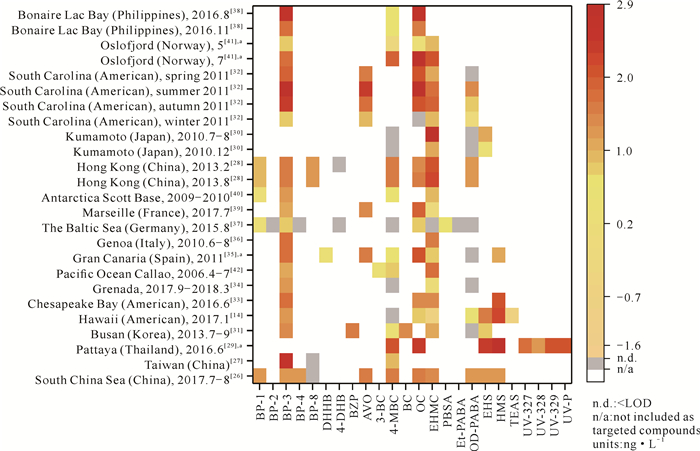
2.1.1 海水中OUVs的时空分布目前已经在海水中开展OUVs分析研究的海域主要分布在亚洲(中国、泰国、日本等)、美洲(美国、格林纳达、秘鲁等)、欧洲(西班牙、意大利、挪威等)及南极洲,包括部分人类活动影响较大的近岸海域[14, 16-42],也包括少量远离人类活动的开阔大洋(太平洋[42])(图 3)。在24种被检测分析的OUVs中,BP-3、EHMC、4-MBC、OC、OD-PABA检测率(开展分析的研究数量/研究总数)分别为88%、84%、72%、69%、58%,检出率(检测到的研究数量/开展分析的研究数量)分别为100%、95%、78%、94%、53%,均较高。苯并三唑类OUVs因亲脂性强,在海水中溶解性小,所以对其开展检测分析的研究相对较少。
|
| a indicates the median concentrations of OUVs in the seawater from different sampling positions in a sea area were used. 图 3 OUVs在全球海水中的时空分布 Fig.3 Spatial and temporal distribution of OUVs in the global ocean |
不同区域海水中OUVs的组成具有明显的区域特异性。例如,中国南海[26]、台湾[27]和香港[28]海域主要受到BP-3、4-MBC、EHMC和OD-PABA的污染,而泰国芭堤雅[29]受到苯并三唑类OUVs (UV-327、UV-328、UV-329和UV-P)的污染较为严重。在美国南卡罗来纳州[32]、荷兰博内尔岛拉克湾[38](8月)和中国台湾[27]海域,海水中的BP-3浓度均高于180 ng·L-1,而在德国波罗的海[37]小于8.30 ng·L-1。在被调研的25个海域中,荷兰博内尔岛拉克湾[38](8月)、美国南卡罗来纳州[32](夏、秋)和泰国芭堤雅[29](6月)受OUVs污染最为严重,OUVs平均浓度分别为294.60、219.70、198.60 ng·L-1;而格林纳达[34]和德国波罗的海[37]受到的OUVs污染较轻,OUVs平均浓度分别仅为5.18 ng·L-1和2.16 ng·L-1。荷兰博内尔岛拉克湾、美国南卡罗来纳州和泰国均为旅游胜地,例如,泰国在全球旅游国家排名中列于前十[29];博内尔岛拉克湾70%的游客来自美国和荷兰,其使用的防晒霜中OUVs主要添加成分为BP-3和OC[38];美国南卡罗来纳州研究中采样点是夏季的海滩,旅游人数较多[32]。相反,格林纳达处于开放海域,海水的流通性较强,易于将OUVs稀释[34];德国波罗的海采样位点包含娱乐活动较少的海域,防晒霜释放量较少[37]。虽然南极洲受人类活动干扰较小,但在其周边海域仍然检测到4-MBC、BP-3和EHMC等多种OUVs,这可能是南极洲科学研究站工作人员排放生活废水所致[40]。
OUVs在海水中的浓度分布与季节影响下的旅游活动紧密相关。在美国南卡罗来纳州、日本熊本[30]、中国香港[28]和挪威奥斯陆峡湾[41]海域,夏季海水中的OUVs浓度均明显高于其他季节(图 3)。例如,在美国南卡罗来纳州,夏季每种OUVs的浓度为30.20-696.60 ng·L-1,而冬季为ND (未检测到)-12.40 ng·L-1;在日本熊本,水杨酸异辛酯(EHS)和EHMC浓度在夏季时分别为14.50 ng·L-1和750 ng·L-1,而冬季时分别为2.80 ng·L-1和15.70 ng·L-1。这种季节性的差异应该与夏季游客数量大以及因较强的紫外线辐射而使用更多的防晒霜等护肤品相关[28, 30, 32]。而在西班牙大加纳利岛海域,由于四季温差较小,旅游业受季节影响较小,夏季和冬季海水中OUVs浓度则无显著性差异[35]。
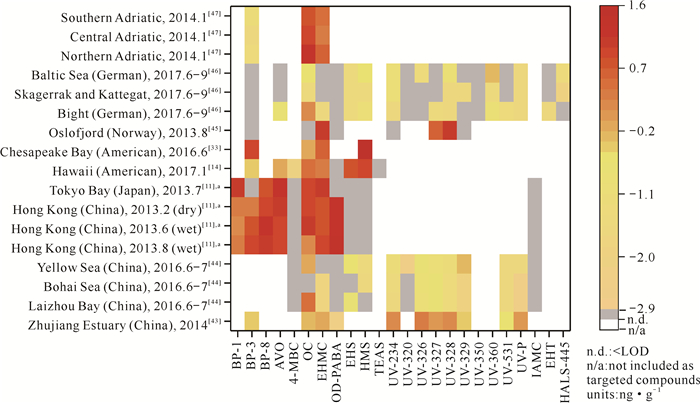
2.1.2 海洋沉积物中OUVs的时空分布目前在海洋沉积物中开展OUVs研究的海域主要分布在中国、日本、美国、德国和挪威等地[11, 14, 33, 43-47](图 4),其中,OC、EHMC、BP-3、OD-PABA、HMS、EHS是检测率最高的6种OUVs,分别为100%、100%、82%、76%、71%和65%。与海水相比,苯并三唑类OUVs的检测率从3%-7%提升到5%-50%,这可能是因为苯并三唑类OUVs具有较大的有机碳标准化分配系数(log Koc>5),较易被沉积物所吸附,人们对其关注度提高[11, 48]。值得注意的是,BP-3在海水中的检出率为100%,而在沉积物中只有64%,说明其在海水中的存在普遍高于沉积物,推测这和BP-3较强的亲水性及较弱的有机碳吸附能力(log Koc=3.1)有关[47]。
|
| a indicates the median concentrations of OUVs in the sediments from different sampling positions in a sea area were used. 图 4 OUVs在全球海洋沉积物中的浓度分布 Fig.4 Concentration distribution of OUVs in the sediments of global ocean |
在海洋沉积物中,OUVs的分布同样具有区域特异性。例如,中国北方(莱州湾、渤海和黄海)[44]的OUVs污染以OC污染为主,而南方香港海域[11]则以OD-PABA为主。总体上,在被研究的15个海域,香港海域OUVs污染最为严重(2、6、8月OUVs浓度之和的平均值为8.94 ng·g-1),而德国海湾、斯卡格拉克海峡和卡特加特海峡、波罗的海[46]海域污染较轻(OUVs浓度之和的平均值分别为0.16、0.03、0.15 ng·g-1)。香港靠近高度工业化及城市化的珠江三角洲,每年有大量悬浮沉积物从珠江口流入香港海域,造成香港海域沉积物中的OUVs含量较高[11]。德国海湾、斯卡格拉克海峡和卡特加特海峡、波罗的海污染源相对较少,例如德国海湾的污染源主要是河流排放,未受到娱乐活动的影响[46]。OUVs在海洋沉积物中的空间分布除受人类活动强度、生活废水处理方式等因素影响外,还和护肤品中OUVs的使用规定有关[47]。例如,在美国护肤品中,HMS最高允许添加量为15%,而在中国和欧盟仅为10%,这可能是美国海域检测到的HMS含量高于欧盟国家海域的原因[49]。
由于沉积物的沉降速率较低,除非通过专门的沉积颗粒物捕集器开展研究方可获取不同时间尺度的样品,否则很难获得理想的样品,因此OUVs在海洋沉积物中的时间分布研究很少。根据仅有的在香港海域开展的季节性/时间分布研究表明,沉积物中OUVs的浓度在雨季和旱季无显著性差异[11]。
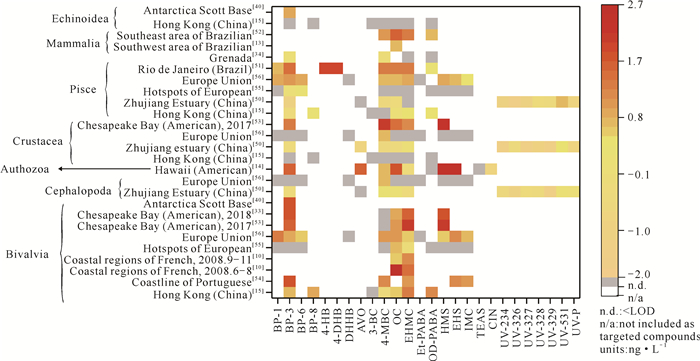
2.1.3 海洋生物中OUVs的富集特征双壳纲(牡蛎、贻贝)、鱼纲、甲壳纲(蟹、对虾)、头足纲(乌贼)、哺乳纲(海豚)、海胆纲、珊瑚纲等多种海洋生物中均有OUVs被检出[10, 13-15, 33, 34, 40, 50-56](图 5)。在被检测出的26种OUVs中,EHMC、BP-3、OC和4-MBC检测率较高,分别为88%、84%、84%和84%。
|
| 图 5 全球海洋生物中OUVs的富集 Fig.5 Enrichment of OUVs in global marine organisms |
OUVs在海洋生物中的分布规律可以归纳为以下两点。
(1) 物种特异性。在中国香港,BP-3、BP-8、OC、EHMC、OD-PABA在双壳纲和鱼纲海洋生物中的浓度分别为3.87-21.48 ng·g-1和0.46-3.15 ng·g-1,而在甲壳纲和海胆纲中均未被检出[15];在欧洲,OUVs在市售双壳纲海洋生物中的浓度为3.30-25.50 ng·g-1,而在甲壳纲和头足纲生物中均未被检出[56]。游泳性动物,如鱼类,具有较强的避害手段,OUVs在其体内普遍被检出,表明OUVs在海洋中存在的广泛性。OUVs浓度分布的物种特异性可能和两个因素有关:①不同物种富集OUVs的能力有差异[14]。例如,EHS、HMS、OC和BP-3在珊瑚体内的浓度显著高于其他生物,这可能是因为珊瑚通过分泌黏液来抵御环境胁迫,而导致黏液成为富集污染物的一个重要途径。②OUVs在不同生物体内的代谢途径和速率有差异。OUVs在生物体内发生降解转化或与内源性代谢物络合,干扰生物体的代谢,例如,EHMC经微生物代谢转化为4-巯基苯甲酸和间甲氧基苯酚[57];BP-3在鲤鱼体内转化为毒性更强的二苯酮-1 (BP-1)、二苯酮-8 (BP-8)等[58];OC与脂质络合,引起珊瑚线粒体功能紊乱[59]。Volpe等[60]利用含有两份不同群落结构的微生物的沉积物降解4-MBC和OD-PABA,发现二者降解速率不同;Zhang等[57]通过培养降解EHMC后的沉积物中的微生物,发现EHMC的降解速率和细菌属Anaerolineaceae、Burkholderiaceae的含量具有显著相关性。
(2) 组织特异性。OUVs在海洋生物体中的分布具有组织特异性。通常,肝脏富集毒物的能力高于其他器官,如在巴西瓜纳巴拉湾的鲻鱼肝脏中,4-DHB、4-HB的浓度约为100 ng·g-1,而在肌肉和鳃中其浓度仅为10 ng·g-1[51]。肝脏中高水平的OUVs可能是因为肝脏是代谢的主要器官,外源污染物易于在其内部累积富集,如BP-3去甲基化和羟基化主要发生在肝脏中[59],严重干扰海洋生物的肝脏代谢[61];OC和EHMC能够通过调控生物体肝细胞中各种基因(如雌激素受体基因、性别决定基因、抗氧化基因)的表达,破坏肝脏组织[62-64]。OUVs可以通过胎盘在母体和胎儿之间传输,然而,由于胎儿尚未发育成熟、各器官功能不完善、排泄代谢毒物的能力有限,对毒物表现出更强的敏感性[65],如OC、4-MBC和EHMC等被检测出在海豚胎儿体内的浓度高于母体[52]。
海洋生物中OUVs的分布同样具有时空差异。以研究最多的双壳纲为例,在法国沿海,贻贝中OC和EHMC的浓度分别为262.00 ng·g-1和28.80 ng·g-1[10],而在欧洲热点地区(西舍尔特、塔古斯河口和埃布罗三角洲),其浓度降低1-2个数量级[55];在葡萄牙海岸,贻贝中BP-3的浓度为77.83 ng·g-1[54],而在中国香港,其浓度仅为5.50 ng·g-1[15]。同沉积物一样,海洋生物中OUVs随时间变化的研究还很少。仅有的一篇相关研究表明,法国海域由于夏季高温、旅游活动强度大和水体交换能力弱的因素,6-8月贻贝中EHMC和OC的浓度分别为9-11月的2倍和100倍[10]。
2.2 OUVs对海洋生物的生态风险评价 2.2.1 PNEC值的计算表 1列出了基于AF法计算得出的OUVs的PNECs值。经计算,OC、EHMC、OD-PABA、EHS和AVO的PNECs值分别为61.00、7.00、6.00、236.00、184.00 ng·L-1。
| 化合物 Compound |
受试生物 Test organisms |
暴露时间/(h) Exposure time/(h) |
LC50/EC50/(μg·L-1) | AF值 AF values |
PNEC/(ng·L-1) |
| OC | Artemia salina | 48.0 | 610 (LC50)[66] | 10 000 | 61 |
| Paracentrotus lividus | 48.0 | 737 (EC50)[67] | |||
| Ficopomatus enigmaticus | 48.0 | 14 740 (EC50)[68] | |||
| EHMC | Isochrysis galbana | 72.0 | 75 (EC50)[69] | 10 000 | 7 |
| Aliivibrio fischeri | 0.5 | 1 060 (EC50)[68] | |||
| F.enigmaticus | 48.0 | 2 810 (EC50)[68] | |||
| OD -PABA | I.galbana | 72.0 | 59 (EC50)[67] | 10 000 | 6 |
| Mytilus galloprovincialis | 48.0 | 130 (EC50)[67] | |||
| P.lividus | 48.0 | 279 (EC50)[67] | |||
| EHS | Artemia salina | 48.0 | 2 360 (LC50)[66] | 10 000 | 236 |
| AVO | A.salina | 48.0 | 1 840 (LC50)[66] | 10 000 | 184 |
| Phaeodactylum tricornutum | 72.0 | 9 450 (EC50)[68] | |||
| F.enigmaticus | 48.0 | 9 890 (EC50)[68] |
和AF相比,SSD的表现几乎不会受到数据标准偏差的影响[70],因此具有较好的稳定性,并且随着理学数据的增加,计算得出的PNEC值能够更好地代表生物受干扰时的污染物浓度水平。然而,对于大部分OUVs而言,关于海洋生物的毒理实验较少,因此相关的毒理学数据较为有限。本文选择了毒理学数据相对较多的BP-3 (共有10个数据)和4-MBC (共有8个数据),利用SSD曲线计算其PNEC值(图 6)。经计算,BP-3和4-MBC的PNECs值分别为0.60 μg·L-1和30.72 μg·L-1。

|
| 图 6 BP-3 (a)和4-MBC (b)的物种敏感度分布曲线 Fig.6 Species sensitivity distribution curves of BP-3 (a) and 4-MBC (b) |
2.2.2 OUVs对海洋生物的生态风险评价
基于以上PNECs值,利用风险商法对世界各地已开展研究海域海水中的BP-3、EHMC、OC、OD-PABA、AVO及EHS进行生态风险评价。如表 2所示,BP-3、EHMC、OC、OD-PABA、AVO和EHS分别在37.5%、87.0%、100.0%、44.4%、60.0%和60.0%的海域对海洋生物具有中高风险,而4-MBC在所有海域对海洋生物均无风险。其中,EHMC在87%的海域RQ值为0.10-107.10,表明该污染物在海洋环境中高毒性的普遍性。不同OUVs对海洋生物的风险差异一方面取决于其环境浓度差异,高浓度必然导致生物体内污染物的富集加剧;另一方面取决于其毒性差异。研究证明高脂溶性化合物,如EHMC (log Kow=5.8)、OC (log Kow=6.88)等,更易在生物体内富集[71]。此外,OUVs的毒性受控于其环境行为,如EHMC光降解产生毒性更强的4-MBA[72],OC较强的抗氧化能力使其在环境中不易降解[73]。
| 海域 Sea area |
BP-3 | EHMC | OC | OD-PABA | 4-MBC | AVO | EHS | |||||||||||||
| EMCa/(ng·L-1) | RQ | EMC/(ng·L-1) | RQ | EMC/(ng·L-1) | RQ | EMC/(ng·L-1) | RQ | EMC/(ng·L-1) | RQ | EMC/(ng·L-1) | RQ | EMC/(ng·L-1) | RQ | |||||||
| South China Sea (China)[26] |
12.86 | 0.02b | 21.53 | 3.10d | 25.00 | 0.41d | 16.23 | 2.70d | 25.99 | 0.00a | 16.27 | 0.09b | 16.18 | 0.07b | ||||||
| Taiwan (China)[27] |
181.81 | 0.30c | 5.83 | 0.00a | ||||||||||||||||
| Hong Kong (China)[28] |
42.44 | 0.07b | 139.36 | 19.90d | 36.23 | 0.59c | 22.30 | 3.70d | 35.17 | 0.00a | ||||||||||
| Pattaya (Thailand)[29] |
249.00 | 4.08d | 104.00 | 0.00a | 682.00 | 2.89d | ||||||||||||||
| Kumamoto (Japan)[30] |
750.00 | 107.10d | ND | \ | ND | \ | ||||||||||||||
| Busan (Korea)[31] |
28.76 | 0.05b | 16.80 | 2.40d | ND | \ | 2.69 | 0.00a | ||||||||||||
| South Carolina (American)[32] |
696.60 | 1.16d | 84.60 | 12.10d | 681.00 | 11.16d | 30.20 | 5.00d | 217.00 | 1.18d | ||||||||||
| Hawaii (American)[14] |
19.86 | 0.03b | 0.24 | 0.03b | 6.15 | 0.10b | 3.15 | 0.53c | 0.03 | 0.00a | ND | \ | 144.74 | 0.61c | ||||||
| Chesapeake Bay (American)[33] |
60.62 | 0.10b | 25.04 | 3.60d | 24.84 | 0.41c | 90.24 | 0.38c | ||||||||||||
| Grenada[34] | 19.57 | 0.03b | 1.14 | 0.15c | ND | \ | ND | \ | ||||||||||||
| Pacific Ocean Callao[42] |
13.28 | 0.02b | 39.47 | 5.60d | 6.86 | 0.00a | ||||||||||||||
| Gran Canaria (Spain)[35] |
50.30 | 0.08b | 8.90 | 1.30d | 106.55 | 1.75d | ND | \ | 5.10 | 0.00a | 44.50 | 0.24c | 13.35 | 0.06b | ||||||
| Genoa (Italy)[36] |
61.08 | 0.10b | 37.58 | 5.40d | ||||||||||||||||
| Oslofjord (Norway)[48] |
62.31 | 0.10b | 125.35 | 17.90d | 759.54 | 12.45d | 91.26 | 0.00a | ||||||||||||
| The Baltic Sea (Germany)[37] |
8.20 | 0.01b | ND | \ | 7.90 | 0.13c | ND | \ | ND | \ | ||||||||||
| Bonaire Lac Bay (Philippines)[38] |
412.10 | 0.69c | 466.60 | 7.65d | 5.10 | 0.00a | ||||||||||||||
| Marseille (France)[39] |
16.81 | 0.03b | 0.76 | 0.10b | 80.45 | 1.32d | 30.13 | 0.16c | ||||||||||||
| Antarctica Scott Base[40] |
23.54 | 0.04b | 9.96 | 1.40d | 4.51 | 0.00a | ||||||||||||||
| Note:a indicates 0≤RQ < 0.01, no risk; b indicates 0.01≤RQ < 0.1, low risk; c indicates 0.1≤RQ < 1, moderate risk; d indicates RQ≥1, high risk; ND indicates not detected or concentration below detection limit; \ indicates no risk assessment conducted. | ||||||||||||||||||||
3 结论
OUVs广泛分布于海水、海洋沉积物及生物样品中,其中3种介质中BP-3和EHMC均为检测率最高的两种物质。海洋中OUVs的赋存和时空分布特征与其污染源和降解途径有关。通常,近岸海域由于受到人类活动的影响较大,其浓度水平高于离岸海域;而在时间上,夏季随着抗紫外线产品使用量增加,OUVs释放量也高于其他季节。海洋生物对OUVs的富集存在明显的物种及组织特异性。基于现有的生态毒理学数据,通过风险商法对海水中BP-3、EHMC、OC、OD-PABA、AVO及EHS等6种OUVs进行生态风险评价,从中高风险海域占总研究海域的比例来看,OC和EHMC是对海洋生态健康威胁较大的两种物质,分别在100.0%和87.0%的海域对海洋生物具有中高风险;而4-MBC对海洋生物的威胁最小,其在所有海域均无风险。
为了更全面地评估OUVs在海洋环境中的污染态势及其对海洋生态环境健康的影响,未来需要在以下5个方面继续开展工作:①加强近岸海域OUVs的环境监测。近岸海域是受人类活动影响最大的区域,全方位覆盖近岸海域OUVs的环境监测能够为海洋环境保护政策制定提供强有力的支撑。②开展新型OUVs的海洋环境监测。随着BP-3、EHMC等传统OUVs的禁用,新型OUVs (如UVT-150)取而代之,掌握这些新型OUVs在海洋环境中的分布有助于及时评估其海洋生态风险。③全面开展OUVs对海洋生物的毒理学实验。毒理学数据是生态风险评估的基础,目前,OUVs对海洋生物毒理的研究仍处于较为初级的阶段,部分物质的毒理学数据较少,开展全面的毒理学实验有助于海洋生态风险评估。④调查大气传输对OUVs迁移的影响。现有研究表明大气颗粒物中可以检测到OUVs的存在,但其是否具有长距离迁移的能力值得探讨。⑤研究OUVs的环境行为及其影响因素。如水体溶解性有机碳、颗粒有机碳对OUVs环境行为的影响,沉积物有机碳对OUVs在沉积物和上覆水中分配的影响等。
| [1] |
仝天衡, 杨慧婷, 陈辉辉, 等. 紫外吸收剂在湖泊中的分布及其对底栖动物的毒性效应[J]. 生态毒理学报, 2019, 14(3): 1-17. |
| [2] |
熊松松. 珠江流域淡水水生生物中有机紫外线吸收剂的富集[D]. 北京: 中国科学院大学(中国科学院广州地球化学研究所), 2017.
|
| [3] |
FALFUSHYNSKA H, SOKOLOV E P, FISCH K, et al. Biomarker-based assessment of sublethal toxicity of organic UV filters (ensulizole and octocrylene) in a sentinel marine bivalve Mytilus edulis[J]. Science of the Total Environment, 2021, 798: 149171. DOI:10.1016/j.scitotenv.2021.149171 |
| [4] |
O'DONOVAN S, MESTRE N C, ABEL S, et al. Effects of the UV filter, oxybenzone, adsorbed to microplastics in the clam Scrobicularia plana[J]. Science of the Total Environment, 2020, 733: 139102. DOI:10.1016/j.scitotenv.2020.139102 |
| [5] |
ARAUJO M J, ROCHA R J M, SOARES A M V M, et al. Effects of UV filter 4-methylbenzylidene camphor during early development of Solea senegalensis Kaup, 1858[J]. Science of the Total Environment, 2018, 628/629: 1395-1404. DOI:10.1016/j.scitotenv.2018.02.112 |
| [6] |
THIA E, CHOU P H, CHEN P J. In vitro and in vivo screening for environmentally friendly benzophenone-type UV filters with beneficial tyrosinase inhibition activity[J]. Water Research, 2020, 185: 116208. DOI:10.1016/j.watres.2020.116208 |
| [7] |
欧盟降低防晒剂二苯酮-3最大允许使用浓度[J]. 日用化学品科学, 2017, 40(4): 21.
|
| [8] |
CHISVERT A, SALVADOR A. UV filters in sunscr-eens and other cosmetics.Regulatory aspects and analytical methods[J]. Analysis of Cosmetic Products, 2007, 182(34): 83-120. |
| [9] |
RAFFA R B, PERGOLIZZI J V, TAYLOR R, et al. Sunscreen bans: coral reefs and skin cancer[J]. Journal of Clinical Pharmacy and Therapeutics, 2019, 44(1): 134-139. DOI:10.1111/jcpt.12778 |
| [10] |
BACHELOT M, LI Z, MUNARON D, et al. Organic UV filter concentrations in marine mussels from French coastal regions[J]. Science of the Total Environment, 2012, 420: 273-279. DOI:10.1016/j.scitotenv.2011.12.051 |
| [11] |
TSUI M M P, LEUNG H W, KWAN B K Y, et al. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in marine sediments in Hong Kong and Japan[J]. Journal of Hazardous Materials, 2015, 292: 180-187. DOI:10.1016/j.jhazmat.2015.03.025 |
| [12] |
RAMOS S, HOMEM V, ALVES A, et al. A review of organic UV-filters in wastewater treatment plants[J]. Environment International, 2016, 86: 24-44. DOI:10.1016/j.envint.2015.10.004 |
| [13] |
GAGO-FERRERO P, ALONSO M B, BERTOZZI C P, et al. First determination of UV filters in marine mammals.Octocrylene levels in Franciscana dolphins[J]. Environmental Science & Technology, 2013, 47: 5619-5625. |
| [14] |
MITCHELMORE C L, HE K, GONSIOR M, et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii[J]. Science of the Total Environment, 2019, 670: 398-410. DOI:10.1016/j.scitotenv.2019.03.034 |
| [15] |
SANG Z, LEUNG K S. Environmental occurrence and ecological risk assessment of organic UV filters in marine organisms from Hong Kong coastal waters[J]. Science of the Total Environment, 2016, 566-567: 489-498. DOI:10.1016/j.scitotenv.2016.05.120 |
| [16] |
孙乾航, 郑欣, 闫振广, 等. 淡水环境中短链氯化石蜡的预测无效应浓度及生态风险评估[J]. 生态毒理学报, 2020, 15(1): 256-264. |
| [17] |
TSUI M M P, LEUNG H W, LAM P K S, et al. Seasonal occurrence, removal efficiencies and preliminary risk assessment of multiple classes of organic UV filters in wastewater treatment plants[J]. Water Research, 2014, 53: 58-67. DOI:10.1016/j.watres.2014.01.014 |
| [18] |
雷炳莉, 文育, 王艺陪, 等. 不同评估方法得出的五氯酚的PNEC值的比较研究[J]. 环境科学, 2013, 34(6): 2335-2343. |
| [19] |
DU M, WEI D B, TAN Z W, et al. The potential risk assessment for different arsenic species in the aquatic environment[J]. Journal of Environmental Sciences, 2015, 27: 1-8. DOI:10.1016/j.jes.2014.03.006 |
| [20] |
王雪梅, 胡金朝, 刘国, 等. 基于商值法的镧水生态风险评价方法研究及应用[J]. 生态毒理学报, 2022, 17(1): 290-298. |
| [21] |
VAN LEEUWEN K V L. Technical guidance docume-nt on risk assessment in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation (EC) No 1488/94 on risk assessment for existing substances, and directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market[Z]. 1996.
|
| [22] |
VIGHI M, FINIZIO A, VILLA S. The evolution of the environmental quality concept: from the US EPA red book to the European water framework directive[J]. Environmental Science & Pollution Research, 2006, 13(1): 9-14. |
| [23] |
林荣华, 姜辉, 王猛, 等. 物种敏感度分布(SSD)方法在农药环境风险评估中的应用[J]. 生态毒理学报, 2017, 12(4): 110-118. |
| [24] |
MAO F J, YOU L H, REINHARD M, et al. Occurrence and fate of benzophenone-type UV filters in a tropical urban watershed[J]. Environmental Science & Technology, 2018, 52(7): 3960-3967. |
| [25] |
TSUI M M P, LEUNG H W, WAI T C, et al. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries[J]. Water Research, 2014, 67: 55-65. DOI:10.1016/j.watres.2014.09.013 |
| [26] |
TSUI M M P, CHEN L G, HE T T, et al. Organic ultraviolet (UV) filters in the South China sea coastal region: environmental occurrence, toxicological effects and risk assessment[J]. Ecotoxicology and Environmental Safety, 2019, 181: 26-33. DOI:10.1016/j.ecoenv.2019.05.075 |
| [27] |
KU P C, LIU T Y, LEE S H, et al. An environmentally friendly strategy for determining organic ultraviolet filters in seawater using liquid-phase microextraction with liquid chromatography-tandem mass spectrometry[J]. Environmental Science and Pollution Research, 2020, 27: 9818-9825. DOI:10.1007/s11356-020-07599-6 |
| [28] |
LI A J, SANG Z Y, CHOW C H, et al. Environmental behavior of 12 UV filters and photocatalytic profile of ethyl-4-aminobenzoate[J]. Journal of Hazardous Materials, 2017, 337: 115-125. DOI:10.1016/j.jhazmat.2017.04.067 |
| [29] |
JUKSU K, LIU Y S, ZHAO J L, et al. Emerging contaminants in aquatic environments and coastal waters affected by urban wastewater discharge in Thailand: an ecological risk perspective[J]. Ecotoxicology and Environmental Safety, 2020, 204: 110952. DOI:10.1016/j.ecoenv.2020.110952 |
| [30] |
SANKODA K, MURATA K, TANIHATA M, et al. Seasonal and diurnal variation of organic ultraviolet filters from personal care products used along the Japanese coast[J]. Archives of Environmental Contamination and Toxicology, 2015, 68: 217-224. DOI:10.1007/s00244-014-0106-7 |
| [31] |
KIM K Y, EKPEGHERE K I, JEONG H J, et al. Effects of the summer holiday season on UV filter and illicit drug concentrations in the Korean wastewater system and aquatic environment[J]. Environmental Pollution, 2017, 227: 587-595. DOI:10.1016/j.envpol.2017.04.055 |
| [32] |
BRATKOVICS S, WIRTH E, SAPOZHNIKOVA Y, et al. Baseline monitoring of organic sunscreen compounds along South Carolina's coastal marine environment[J]. Marine Pollution Bulletin, 2015, 101(1): 370-377. DOI:10.1016/j.marpolbul.2015.10.015 |
| [33] |
HE K, HAIN E, TIMM A, et al. Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake Bay[J]. Science of the Total Environment, 2019, 650: 3101-3109. DOI:10.1016/j.scitotenv.2018.10.021 |
| [34] |
HORRICKS R A, TABIN S K, EDWARDS J J, et al. Organic ultraviolet filters in nearshore waters and in the invasive lionfish (Pterois volitans) in Grenada, West Indies[J]. PLoS One, 2019, 14(7): e0220280. DOI:10.1371/journal.pone.0220280 |
| [35] |
SÁNCHEZ RODRÍGUEZ A, RODRIGO SANZ M, BETANCORT RODRÍGUEZ J R. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands).An approach to environmental risk assessment[J]. Chemosphere, 2015, 131: 85-90. DOI:10.1016/j.chemosphere.2015.02.054 |
| [36] |
NGUYEN K T N, SCAPOLLA C, DI CARRO M, et al. Rapid and selective determination of UV filters in seawater by liquid chromatography-tandem mass spectrometry combined with stir bar sorptive extraction[J]. Talanta, 2011, 85(5): 2375-2384. DOI:10.1016/j.talanta.2011.07.085 |
| [37] |
FISCH K, WANIEK J J, SCHULZ-BULL D E. Occurrence of pharmaceuticals and UV-filters in riverine run-offs and waters of the German Baltic Sea[J]. Marine Pollution Bulletin, 2017, 124(1): 388-399. DOI:10.1016/j.marpolbul.2017.07.057 |
| [38] |
SCHAAP I, SLIJKERMAN D M E. An environmental risk assessment of three organic UV-filters at Lac Bay, Bonaire, Southern Caribbean[J]. Marine Pollution Bulletin, 2018, 135: 490-495. DOI:10.1016/j.marpolbul.2018.07.054 |
| [39] |
LABILLE J, SLOMBERG D, CATALANO R, et al. Assessing UV filter inputs into beach waters during recreational activity: a field study of three French Mediterranean beaches from consumer survey to water analysis[J]. Science of the Total Environment, 2020, 706: 136010. DOI:10.1016/j.scitotenv.2019.136010 |
| [40] |
EMNET P, GAW S, NORTHCOTT G, et al. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base[J]. Environmental Research, 2015, 136: 331-342. DOI:10.1016/j.envres.2014.10.019 |
| [41] |
LANGFORD K H, THOMAS K V. Inputs of chemicals from recreational activities into the Norwegian coastal zone[J]. Journal of Environmental Monitoring, 2008, 10(7): 894-898. DOI:10.1039/b806198j |
| [42] |
GOKS∅YR A, TOLLEFSEN K E, GRUNG M, et al. Balsa raft crossing the Pacific finds low contaminant levels[J]. Environmental Science & Technology, 2009, 43(13): 4783-4790. |
| [43] |
PENG X Z, XIONG S S, OU W H, et al. Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China[J]. Journal of Hazardous Materials, 2017, 323: 139-146. DOI:10.1016/j.jhazmat.2016.05.020 |
| [44] |
APEL C, TANG J, EBINGHAUS R. Environmental occurrence and distribution of organic UV stabilizers and UV filters in the sediment of Chinese Bohai and Yellow Seas[J]. Environmental Pollution, 2018, 235: 85-94. DOI:10.1016/j.envpol.2017.12.051 |
| [45] |
LANGFORD K H, REID M J R, FJELD E, et al. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway[J]. Environment International, 2015, 80: 1-7. DOI:10.1016/j.envint.2015.03.012 |
| [46] |
APEL C, JOERSS H, EBINGHAUS R. Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas[J]. Chemosphere, 2018, 212: 254-261. DOI:10.1016/j.chemosphere.2018.08.105 |
| [47] |
COMBI T, PINTADO-HERRERA M G, LARAMAR-TIN P A, et al. Distribution and fate of legacy and emerging contaminants along the Adriatic Sea: a comparative study[J]. Environmental Pollution, 2016, 218: 1055-1064. DOI:10.1016/j.envpol.2016.08.057 |
| [48] |
SETH R, MACKAY D, MUNCKE J. Estimating the organic carbon partition coefficient and its variability for hydrophobic chemicals[J]. Environmental Science & Technology, 1999, 33(14): 2390-2394. |
| [49] |
裘炳毅, 高志红. 现代化妆品科学与技术: 上册[M]. 广州: 中国轻工业出版社, 2016.
|
| [50] |
PENG X Z, FAN Y J, JIN J B, et al. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea[J]. Environmental Pollution, 2017, 225: 55-65. DOI:10.1016/j.envpol.2017.03.035 |
| [51] |
MOLINS-DELGADO D, MUNOZ R, NOGUEIRA S, et al. Occurrence of organic UV filters and metabolites in lebranche mullet (Mugil liza) from Brazil[J]. Science of the Total Environment, 2018, 618: 451-459. DOI:10.1016/j.scitotenv.2017.11.033 |
| [52] |
ALONSO M B, FEO M L, CORCELLAS C, et al. Toxic heritage: maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil[J]. Environmental Pollution, 2015, 207: 391-402. DOI:10.1016/j.envpol.2015.09.039 |
| [53] |
HE K, TIMM A, BLANEY L. Simultaneous determination of UV-filters and estrogens in aquatic invertebrates by modified quick, easy, cheap, effective, rugged, and safe extraction and liquid chromatography tandem mass spectrometry[J]. Journal of Chromatography A, 2017, 1509: 91-101. DOI:10.1016/j.chroma.2017.06.039 |
| [54] |
CASTRO M, FERNANDES J O, PENA A, et al. Occurrence, profile and spatial distribution of UV-filters and musk fragrances in mussels from Portuguese coastline[J]. Marine Environmental Research, 2018, 138: 110-118. DOI:10.1016/j.marenvres.2018.04.005 |
| [55] |
CUNHA S C, FERNANDES J O, VALLECILLOS L, et al. Co-occurrence of musk fragrances and UV-filters in seafood and macroalgae collected in European hotspots[J]. Environmental Research, 2015, 143: 65-71. DOI:10.1016/j.envres.2015.05.003 |
| [56] |
CUNHA S C, TRABALÓN L, JACOBS S, et al. UV-filters and musk fragrances in seafood commercialized in Europe Union: occurrence, risk and exposure assessment[J]. Environmental Research, 2018, 161: 399-408. DOI:10.1016/j.envres.2017.11.015 |
| [57] |
ZHANG P, LU G H, LIU J C, et al. Biodegradation of 2-ethylhexyl-4-methoxycinnamate in river sediments and its impact on microbial communities[J]. Journal of Environmental Sciences, 2021, 104: 307-316. DOI:10.1016/j.jes.2020.12.013 |
| [58] |
CHIRIAC F L, LUCACIU I E, PAUN I, et al. In vivo bioconcentration, distribution and metabolization of Benzophenone-3 (BP-3) by Cyprinus carpio (European Carp)[J]. Foods, 2022, 11(11): 1627. DOI:10.3390/foods11111627 |
| [59] |
STIEN D, CLERGEAUD F, RODRIGUES A M S, et al. Metabolomics reveal that octocrylene accumulates in Pocillopora damicornis tissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction[J]. Analytical Chemistry, 2019, 91(1): 990-995. DOI:10.1021/acs.analchem.8b04187 |
| [60] |
VOLPE A, PAGANO M, MASCOLO G, et al. Biodegradation of UV-filters in marine sediments[J]. Science of the Total Environment, 2017, 575: 448-457. DOI:10.1016/j.scitotenv.2016.10.001 |
| [61] |
ZIARRUSTA H, MIJANGOS L, PICART-ARMADA S, et al. Non-targeted metabolomics reveals alterations in liver and plasma of gilt-head bream exposed to oxybenzone[J]. Chemosphere, 2018, 211: 624-631. DOI:10.1016/j.chemosphere.2018.08.013 |
| [62] |
MENG Q, YEUNG K, CHAN K M. Toxic effects of octocrylene on zebrafish larvae and liver cell line (ZFL)[J]. Aquatic Toxicology, 2021, 236: 105843. DOI:10.1016/j.aquatox.2021.105843 |
| [63] |
NATARAJ B, MAHARAJAN K, MALAFAIA G, et al. Gene expression profiling in liver of zebrafish exposed to ethylhexyl methoxycinnamate and its photoproducts[J]. Science of the Total Environment, 2022, 826: 154046. DOI:10.1016/j.scitotenv.2022.154046 |
| [64] |
CAHOVA J, BLAHOVA J, MARSALEK P, et al. The biological activity of the organic UV filter ethylhexyl methoxycinnamate in rainbow trout (Oncorhynchus mykiss)[J]. Science of the Total Environment, 2021, 774: 145570. DOI:10.1016/j.scitotenv.2021.145570 |
| [65] |
LI Z, TANG Y Q, SONG X, et al. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism[J]. Ecotoxicology and Environmental Safety, 2019, 169: 248-254. |
| [66] |
THOREL E, CLERGEAUD F, JAUGEON L, et al. Effect of 10 UV Filters on the Brine Shrimp Artemia salina and the Marine Microalga Tetraselmis sp.[J]. Toxics, 2020, 8(2): 29. |
| [67] |
PAREDES E, PEREZ S, RODIL R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata[J]. Chemosphere, 2014, 104: 44-50. |
| [68] |
GIRALDO A, MONTES R, RODIL R, et al. Ecotoxicological evaluation of the UV Filters ethylhexyl dimethyl p-aminobenzoic acid and octocrylene using marine organisms Isochrysis galbana, Mytilus galloprovincialis and Paracentrotus lividus[J]. Archives of Environmental Contamination and Toxicology, 2017, 72: 606-611. |
| [69] |
SANCHES M V, OLIVA M, DE MARCHI L, et al. Ecotoxicological screening of UV-filters using a battery of marine bioassays[J]. Environmental Pollution, 2021, 290: 118011. |
| [70] |
SORGOG K, KAMO M. Quantifying the precision of ecological risk: conventional assessment factor method vs.species sensitivity distribution method[J]. Ecotoxicology and Environmental Safety, 2019, 183: 109494. |
| [71] |
DE WOLF W, DE BRUIJN J H M, SEINEN W, et al. Influence of biotransformation on the relationship between bioconcentration factors and octanol-water partition coefficients[J]. Environmental Science & Technology, 1992, 26: 1197-1201. |
| [72] |
GACKOWSKA A, STUDZI$\acute{И}$SKI W, KUDLEK E, et al. Estimation of physicochemical properties of 2-ethylhexyl-4-methoxycinnamate (EHMC) degradation products and their toxicological evaluation[J]. Environmental Science and Pollution Research, 2018, 25: 16037-16049. |
| [73] |
HOPKINS Z R, SNOWBERGER S, BLANEY L. Ozonation of the oxybenzone, octinoxate, and octocrylene UV-filters: reaction kinetics, absorbance characteristics, and transformation products[J]. Journal of Hazardous Materials, 2017, 338: 23-32. |



