鉴于人体血管的解剖特点,经股动脉入路(Transfemoral Approach,TFA)是脑血管疾病介入诊疗的主流选择,但对于Ⅲ型主动脉弓、股/髂动脉严重病变(例如股动脉闭塞、髂总动脉动脉瘤及动脉夹层等)患者,TFA面临着很大困难[1]。桡动脉入路(Transradial Approach,TRA)是一种常用的替代TFA的诊疗入径,自1989年Campeau[2]首次报道经TRA行冠状动脉造影术以来,经桡动脉入路的介入手术得到了迅猛发展。2001年,Matsumoto等[3]最早探索经TRA行神经介入手术,但仅限于全脑血管造影。桡动脉属于体循环二级动脉,因其解剖变异(发育不良、桡/肱动脉迂曲),刺激后较易痉挛,给TRA腔内操作带来一定的困难。Ruzsa等[4]探索了经TRA颈动脉支架的研究,认为成袢或交换技术使得导引导管到位困难,手术操作繁琐复杂,技术难度大,手术时间明显延长,风险亦明显增加;Щаницын等[5]和Schermerhorn等[6]也研究了经TRA颈动脉支架成形术。但与常规TFA相比,桡动脉容易压迫止血,相关并发症少,术后患者舒适度高,无需卧床制动[7],因此临床上具有一定的优势。由于对大脑中动脉支架成形术要求导引导管头端需到达颈动脉C1/2段,而手术所需器材尚不完善,且缺乏专用的导丝、导管及支架系统等原因,经TRA右侧大脑中动脉支架成形术的研究未见报道,因此本研究探讨TRA右侧大脑中动脉支架成形术的可行性、手术技巧和安全性,为临床提供借鉴。
1 资料与方法 1.1 临床资料纳入2019年5月至2021年10月在广西医科大学第二附属医院需要实施右侧大脑中动脉支架成形术的急性缺血性脑血管病患者58例,按照随机数表法分为观察组29例和对照组29例,观察组采用TRA,对照组采用TFA。
入组标准如下。(1)年龄18-75周岁。(2)缺血性脑血管病符合《中国急性缺血性脑卒中诊治指南2018》[8],支架置入标准符合《症状性颅内动脉粥样硬化性狭窄血管内治疗中国专家共识2018》[9],发病1周内入院。(3)术前计算机断层扫描血管造影(Computed Tomographic Angiography,CTA)或数字减影血管造影(Digital Subtraction Angiography,DSA)证实为症状性大脑中动脉局限性狭窄:①70%≤狭窄率≤99%[参照采用华法林-阿司匹林症状性颅内动脉病变(WASID)试验诊断标准,狭窄率=(1-狭窄部位的血管管腔直径/近心端正常血管管腔直径)×100%];②狭窄长度≤15 mm;③计算机断层扫描灌注成像(Computed Tomography Perfusion,CTP)或者磁共振灌注成像(Perfusion-Weighted Imaging,PWI)出现以下情况——达峰时间(TTP)延长、脑血流量(CBF)减少、平均通过时间(MTT)正常或延长、脑血容量(CBV)正常或轻度减少[10]; ④同时满足以下四条中的任意一条:(a)同侧大脑后动脉发育差,同侧大脑前动脉需向同侧大脑中、后动脉代偿供血;(b)同侧大脑前动脉A1段发育差,同侧大脑后动脉需向同侧大脑前、中动脉代偿供血;(c)同侧大脑前动脉和大脑后动脉同时发育差,大脑中动脉需向大脑前动脉、后动脉代偿供血;(d)同侧大脑前动脉和大脑后动脉发育好,正规两种抗血小板药物+积极控制危险因素治疗患者症状仍发作或者家属强烈要求血管成形术,不同意观察该方案治疗效果。(4)患者至少有1个以上的动脉粥样硬化危险因素:年龄、卒中家族史、高血压、糖尿病、高脂血症、吸烟史等。(5)大脑中动脉支架成形术术后遵医嘱口服抗血小板药物、降脂药物治疗,积极配合控制血压、血糖等。(6)观察组Allen试验阴性。
排除标准如下。(1)预计脑梗死后遗留严重神经功能障碍,美国国立卫生研究院卒中量表(National Institute of Health Stroke Scale,NIHSS)≥15分。(2)术侧大脑中动脉狭窄段呈锐角,或狭窄段近端正常血管管径<2 mm,或狭窄段远端弥漫性狭窄、先天性发育不良。(3)非动脉粥样硬化性动脉狭窄。(4)术前新发脑梗死时间≥14 d,且新梗死面积大于同侧大脑中动脉供血区2/3,且无对侧大脑中动脉、椎基底动脉中重度狭窄。(5)合并心房纤颤、风湿性心脏瓣膜病等可能存在心源性卒中患者。(6)严重全身系统性疾病,或血小板计数<1.0×1011/L,或血小板计数>4.5×1011/L,或凝血功能障碍,或具有出血倾向,或对阿司匹林、氯吡格雷、替格瑞洛过敏或不能耐受者(例如:活动性消化道溃疡、出血)。(7)6个月内有脑出血病史。(8)不能耐受全麻手术或有全麻手术禁忌。(9)本次就诊发病前改良RANKIN量表(modified Rankin Scale,mRS)≥3分,术后不能配合控制好血压、血糖、戒烟等患者。本研究已经通过本医院伦理监察委员会审查,所有参加研究的患者均充分了解了本研究的目的及相关注意事项,患者本人或其家属已签署知情同意书。
1.2 治疗方法 1.2.1 药物治疗2组患者术前术后均给予氯吡格雷[波立维,国药准字J20180029,赛诺菲(杭州)制药有限公司生产,75 mg/片]75 mg,口服,每天1次;阿司匹林肠溶片[拜阿司匹灵,国药准字J20130078,Bayer HealthCare Manufacturing S.r.l.生产(拜耳医药保健有限公司分装),100 mg/片] 100 mg,口服,每天1次;阿托伐他汀钙片(立普妥,国药准字H20051408,辉瑞制药有限公司生产,20 mg/片)20 mg,口服,每天1次。术前至少口服≥7 d,术后口服治疗6个月,6个月后停用氯吡格雷,继续口服阿司匹林肠溶片100 mg,每天1次。2组患者术前术后均严格控制血压、血糖(糖化血红蛋白<7%,餐后血糖水平<10 mmol/L)、戒烟等治疗。
1.2.2 手术治疗(1) 观察组:在全身麻醉下选用改良Seldinger穿刺法进行桡动脉插管,如果桡动脉波动差或无搏动,改为肱动脉插管,置入6F动脉鞘后进行全身肝素化处理(1 mg/kg)。将6F导引导管置入右侧颈内动脉恰当位置,置入方法有两种,第一种为同轴技术,第二种为交换技术。①同轴技术:根据右侧颈动脉与锁骨下动脉的解剖关系,将右侧颈动脉分为外侧型、内侧型、外旋型、内旋型。外侧型、内侧型右侧颈动脉使用泥鳅导丝+6F导引导管+120 cm猪尾导管(4F,外径1.35 mm)同轴上行,在路径图导引下利用猪尾导管尾端勾到右侧颈动脉,上行泥鳅导丝到颈动脉远端或者颈外动脉,固定猪尾导管后顺势上行6F导引导管。外旋型、内旋型右侧颈动脉使用泥鳅导丝+6F导引导管直接上行(必要时将泥鳅导丝头端塑形成猪尾巴形)。②交换技术:先将SIMMONS 2型造影导管头端置于右侧颈动脉,路径图导引下将长交换导丝(0.035 inch,260 cm)留置在颈总动脉或颈外动脉,缓慢推进6F导引导管。上述技术如图 1所示。选取目标血管正常直径80%的PTA球囊导管(Gate-way,国械注进20193032148,波士顿科学公司生产)预扩张,通过Excelsior XT-27微导管(国械注进20153031727,美国史赛克公司生产)置入合适型号(支架直径大于目标近端血管0.5-1.0 mm,支架需覆盖病变两端≥3 mm)的颅内支架系统Neuroform EZ[国械注进20173130608,史赛克(北京)医疗器械有限公司],支架置入后即刻进行造影,测量残余狭窄情况,如残余狭窄率>30%,再次进行PTA球囊导管扩张成形,术后桡动脉压迫器压迫止血。

|
| 图 1 6F导引导管置入右侧颈动脉的操作方法 Fig. 1 Operation method of inserting 6F catheter into the right internal carotid artery |
(2) 对照组:在全身麻醉下选用改良Seldinger穿刺法进行股动脉插管,置入6F动脉鞘后进行全身肝素化处理(1 mg/kg)。根据主动脉弓Myla分型[11],利用同轴技术[Ⅲ型主动脉弓:6F导引导管+多功能导管,或6F导引导管+VTK造影导管(国械注进20173031671,库克公司Cook Incorporated生产);Ⅰ型或Ⅱ型主动脉弓:6F导引导管+多功能导管]将6F导引导管置入右侧颈动脉恰当位置,其他手术操作同观察组,术后股动脉穿刺处用Exoseal封堵器封堵止血。2组患者术后行旋转断层重组技术获得CT样图像(digital subtraction angiography laboratory with inbuilt CT,Dyna CT),扫描判断是否出现颅内出血。该项手术由同一术者完成,且术者有≥50例大脑中动脉支架置入经验。
1.3 观察指标记录2组患者导引导管到位情况、支架置入成功率、手术并发症、路径差异相关并发症、手术操作时间、射线投射时间、术后住院天数和手术费用。
1.4 统计学方法正态性检验方法采用K-S检验法,符合正态分布的计量资料采用均数±标准差(x±s)表示,两组间均数比较用t检验;计数资料以频数和率表示,组间比较采用卡方检验或Fisher精确概率法。所有数据通过SPSS 25.0统计学软件进行分析处理,P<0.05表示差异有统计学意义。
2 结果与分析 2.1 2组患者基本情况58例患者中女性人数是男性的1.52倍,74.14%的患者合并有高血压,36.21%的患者合并有糖尿病,68.97%的患者合并有高脂血症。术前大脑中动脉狭窄率为(82.9±11.6)%,术后残存狭窄率为(19.8±8.2)%。如表 1所示,2组患者性别、年龄、血管病危险因素、主动脉弓型、大脑中动脉术前术后狭窄程度等差异无统计学意义。
| 项目 Item |
观察组 Observation group (n=29) |
对照组 Control group(n=29) |
t或 |
P | |
| Male (23,%) | 11(37.93) | 12(41.38) | 0.072 | 0.788 | |
| Age (years, x±s) | 61.25±7.94 | 62.09±8.63 | 0.801 | 0.186 | |
| Risk factors of vascular disease (cases,%) | Hypertension | 23(79.31) | 20(68.97) | 1.096 | 0.895 |
| Diabetes | 10(34.48) | 11(37.93) | |||
| Hyperlipidemia | 19(65.52) | 21(72.41) | |||
| Hyperhomocysteinemia | 5(17.24) | 8(27.59) | |||
| Current smoking | 7(24.14) | 6(20.69) | |||
| Aortic arch type | Type Ⅰ | 8(27.59) | 8(27.59) | 0.099 | 0.952 |
| Type Ⅱ | 12(41.38) | 13(44.83) | |||
| Type Ⅲ | 9(31.03) | 8(27.58) | |||
| Preoperative stenosis rate of middle cerebral artery (x±s) | (84.9±11.6)% | (81.5±10.2)% | 0.861 | 0.508 | |
| Postoperative residual stenosis rate (x±s) | (20.2±6.6)% | (19.4±10.7)% | 0.802 | 0.314 |
|
2.2 2组患者术后情况
58例患者置入支架58枚,这些支架成形术均取得成功。其中,观察组1例患者无触及桡动脉波动,改成肱动脉入径;2例患者Ⅱ型主动脉弓上行6F导引导管困难,改成TFA。观察组导引导管到位成功率93.10%(27/29),27例中采用同轴技术上行导引导管23例(图 2),成功率85.19%,采用交换技术上行导引导管4例,同轴技术优势明显。对照组导引导管到位成功率100%,2组手术成功率的比较差异无统计学意义(

|
| (a) MRI showed right hemisphere hypoperfusion cerebral infarction; (b) PWI showed right hemisphere hypoperfusion; (c) DSA showed type Ⅲ aortic arch with external rotation right carotid artery; (d) DSA showed external rotation right carotid artery direct guide wire superselective entry into right carotid artery; (e) DSA showed no residual stenosis after stenting of right middle cerebral artery; (f) After 6 months, DSA showed that there was no residual stenosis after stenting of the right middle cerebral artery; (g) After 12 months, DSA showed that there was no residual stenosis after stenting of the right middle cerebral artery. 图 2 采用同轴技术上行导引导管行右侧大脑中动脉支架成形术1例 Fig. 2 One case of right middle cerebral artery stenting using coaxial technology with ascending catheter guidance |
2组患者在术后24 h内共出现并发症5例。其中,观察组出现颈动脉C2段创伤性夹层1例,给予补救性Wallstent颈动脉支架(波士顿单轨型颈动脉支架,7 mm×40 mm)置入;术后颅内出血死亡1例(术后高灌注或者球囊扩张撕破血管,如图 3所示),未出现桡动脉出血或闭塞。对照组术后出现穿支病变导致脑梗死2例,股动脉穿刺处出血1例。2组患者因路径差异引起的并发症情况如下:观察组3.70%(1/27),对照组3.45%(1/29),差异无统计学意义(
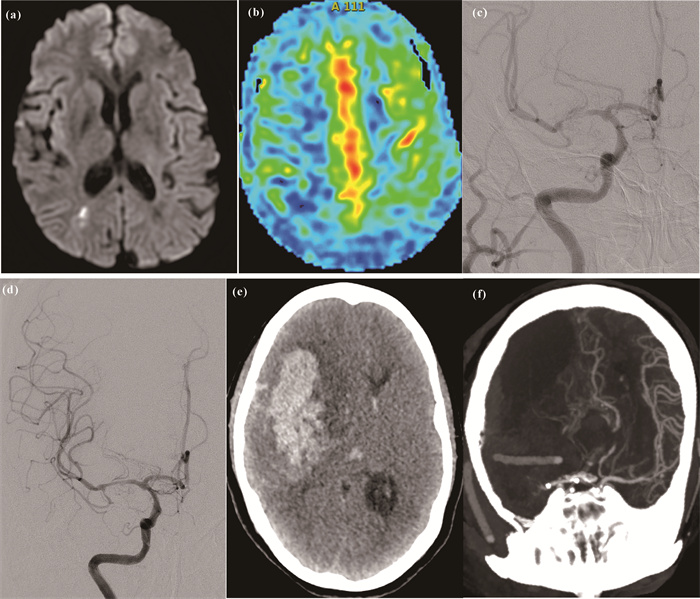
|
| (a) MRI showed hypoperfusion cerebral infarction in the right hemisphere; (b) PWI showed hypoperfusion in the right hemisphere; (c) DSA showed severe stenosis of the right middle cerebral artery; (d) DSA showed no residual stenosis after stenting of the right middle cerebral artery; (e) CT showed intracerebral hemorrhage in the right hemisphere after operation; (f) CTA showed massive cerebral infarction in the right hemisphere with right middle cerebral artery occlusion. 图 3 右侧大脑中动脉支架成形术术后脑出血1例 Fig. 3 One case of cerebral hemorrhage after right middle cerebral artery stenting surgery |
2.3 2组患者其他观察指标的比较
2组患者手术操作时间、射线投射时间和手术费用的比较差异无统计学意义。如表 2所示,根据主动脉弓型的亚组分析,观察组Ⅲ型主动脉弓患者手术操作时间、射线投射时间均短于对照组,差异有统计学意义。观察组术后住院天数(d)少于对照组,差异有统计学意义。2组患者手术费用的比较差异无统计学意义。
| 组别 Group |
总人数 n |
手术操作时间/min Operation time/min |
射线投射时间/min Ray projection time/min |
手术费用/万元 Surgical expenses /ten thou-sand yuan |
术后住院天数/d Postoperative hospitalization days/d |
|||||||
| Ⅰ型 Type Ⅰ |
Ⅱ型 Type Ⅱ |
Ⅲ型 Type Ⅲ |
总计 Total |
Ⅰ型 Type Ⅰ |
Ⅱ型 Type Ⅱ |
Ⅲ型 Type Ⅲ |
总计 Total |
|||||
| Observation group | 27 | 73.2±18.6 | 75.4±20.8 | 73.8±15.3 | 74.1±16.5 | 21.4±6.8 | 23.7±7.1 | 22.1±5.5 | 22.6±4.9 | 5.7±0.6 | 2.8±0.6 | |
| Control group | 29 | 71.9±20.8 | 76.2±16.3 | 89.5±14.4 | 78.3±12.7 | 20.9±8.4 | 21.2±6.9 | 28.4±8.3 | 24.7±7.6 | 5.9±0.8 | 4.2±0.8 | |
| t | 0.146 | 0.604 | 0.192 | 0.187 | 0.206 | 0.472 | 0.117 | 0.635 | 0.582 | 0.230 | ||
| P | 0.337 | 0.212 | 0.000 | 0.901 | 0.180 | 0.537 | 0.000 | 0.174 | 0.212 | 0.002 | ||
3 讨论
《症状性颅内动脉粥样硬化性狭窄血管内治疗中国专家共识2018》[9]建议对于症状性颅内动脉粥样硬化性狭窄(Intracranial Atherosclerotic Stenosis,ICAS)患者狭窄率≥70%,且强化药物治疗无效或脑侧支循环代偿不良时行血管内治疗,置入自膨式支架是ICAS治疗的重要方式[6]。目前,TFA是脑血管介入诊疗的主流选择。因股动脉位置固定、粗大,容易穿刺,是颅内外血管介入治疗的首选手术入路。但股动脉穿刺后易出现穿刺点血肿、皮下或腹膜后出血、假性动脉瘤、动静脉瘘等并发症,且有些患者因股动脉/髂动脉闭塞、主动脉迂曲等原因,使得TFA面临很大困难[1]。因此,经TRA可能是一个好的选择方案[12, 13]。
本研究根据猪尾导管的尾端形态,以及右侧颈动脉与锁骨下动脉的解剖关系,对外侧型、内侧型右侧颈动脉患者,在路径图导引下利用猪尾导管尾端勾到右侧颈动脉,上行泥鳅导丝后顺势上行导引导管。对于外旋型、内旋型右侧颈动脉患者,将泥鳅导丝头端塑形成猪尾巴形后,在路径图下进入右侧颈动脉或颈外动脉,直接上行导引导管。观察组2例患者上行导引导管困难的原因在于头臂干分出右颈动脉和右锁骨下动脉的开口过低,右颈动脉与头臂干夹角为锐角,推送导引导管时导管易滑落到心腔,最终改成TFA。
本研究发现,经TFA和TRA行右侧大脑中动脉支架成形术,2组患者的手术操作时间、射线投射时间的比较无显著差异,但对于Ⅲ型主动脉弓患者,经TRA优势明显,其手术操作时间、射线投射时间均显著缩短。既往对Ⅲ型主动脉弓患者置入颈动脉支架的研究也发现该类患者支架置入困难[14, 15]。对于Ⅲ型主动脉弓患者,经TFA上行导引导管时,由于右颈动脉与头臂干夹角多为锐角,导引导管在血管转弯处易牵拉血管壁,增加了斑块脱落导致脑栓塞或者下肢栓塞的概率[16, 17]。采用本研究中的手术技巧,同轴系统支持力较强,一方面便于导引导管跟进,另一方面也减少导引导管在血管转弯处对血管壁切割,减少脑栓塞或者下肢栓塞的风险,缩短手术时间,同时提高手术成功率。
本研究也发现,经TRA行右侧大脑中动脉支架成形术后,患者术后无需卧床,住院时间明显缩短。由于经TFA术后使用股动脉封堵器,而经TRA术后使用桡动脉压迫器,总体手术费用无明显差异。与经TFA相比,经TRA介入治疗,具有独特的优势:(1)桡动脉位置比较表浅,容易压迫,出血并发症少;(2)术后即刻拔管,有效避免血栓形成;(3)穿刺点附近无大的静脉及神经,不易损伤静脉和神经;(4)术后患者无需卧床,在提高患者生活质量的同时,避免了下肢深静脉血栓形成;(5)无需使用股动脉封堵器、缝合器或闭合器[18, 19]。但经TRA介入治疗仍存在弊端:(1)桡动脉直径较细,通过导引导管时易出现桡动脉痉挛;(2)上肢动脉分支血管较多,易变异或扭曲,部分患者导引导管上行困难;(3)目前无桡动脉专用行脑血管疾病介入手术的器材,经TFA介入器材相对粗大,限制了其在经TRA脑血管疾病介入的诊疗[2, 20]。
本课题组发现,经TRA行右侧大脑中动脉支架成形术有很高的成功率,术后患者恢复快,术后即可下床活动。对于Ⅲ型主动脉弓患者采用该项方案更有优势,但术中需要根据右侧颈动脉与锁骨下动脉的解剖关系技巧性地选择同轴技术或交换技术上行导引导管。对于无桡动脉和肱动脉搏动的患者,或者需要使用大口径导管时,经TFA仍是首选方法[21, 22]。TRA在脑血管疾病诊治领域的应用尚未广泛推广,其手术难度较TFA对术者要求更高。然而,随着微创医疗的不断发展,TRA未来将有广阔的发展空间,但需要专用介入器械的研发与探索[23, 24]。
| [1] |
CESARO A, MOSCARELLA E, GRAGNANO F, et al. Transradial access versus transfemoral access: a comparison of outcomes and efficacy in reducing hemorrhagic events[J]. Expert Review of Cardiovascular Therapy, 2019, 17(6): 435-447. DOI:10.1080/14779072.2019.1627873 |
| [2] |
CAMPEAU L. Percutaneous radial artery approach for coronary angiography[J]. Catheterization and Cardiovascular Diagnosis, 1989, 16(1): 3-7. DOI:10.1002/ccd.1810160103 |
| [3] |
MATSUMOTO Y, HONGO K, TORIYAMA T, et al. Transradial approach for diagnostic selective cerebral angiography: results of a consecutive series of 166 cases[J]. American Journal of Neuroradiology, 2001, 22(4): 704-708. |
| [4] |
RUZSA Z, NEMES B, PINTÉR L, et al. A randomised comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR (RADial access for CARotid artery stenting) study[J]. Eurointervention: Journal of Europcr in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, 2014, 10(3): 381-391. DOI:10.4244/EIJV10I3A64 |
| [5] |
ЩАНИЦЫН И Н, ШАРАФУТДИНОВ М Р, ЯКУ-БОВ Р А, 等. Трансрадиалвный доступпри каротидном стентировании[J]. Ангиология И Сосудистая Хирургия, 2018, 24(2): 114-122. |
| [6] |
SCHERMERHORN M L, LIANG P, ELDRUP-JORGENSEN J, et al. Association of transcarotid artery revascularization vs transfemoral carotid artery stenting with stroke or death among patients with carotid artery stenosis[J]. JAMA, 2019, 322(23): 2313-2322. DOI:10.1001/jama.2019.18441 |
| [7] |
ZALOCAR L A D, DOROSZUK G, GOLAND J. Transradial approach and its variations for neurointerventional procedures: literature review[J]. Surgical Neurology International, 2020, 11: 248. DOI:10.25259/SNI_366_2020 |
| [8] |
中华医学会神经病学分会, 中华医学会神经病学分会脑血管病学组. 中国急性缺血性脑卒中诊治指南2018[J]. 中华神经科杂志, 2018, 51(9): 666-682. |
| [9] |
中国卒中学会, 中国卒中学会神经介入分会, 中华预防医学会卒中预防与控制专业委员会介入学组. 症状性颅内动脉粥样硬化性狭窄血管内治疗中国专家共识2018[J]. 中国卒中杂志, 2018, 13(6): 594-604. |
| [10] |
LAN L F, LENG X Y, IP V, et al. Sustaining cerebral perfusion in intracranial atherosclerotic stenosis: the roles of antegrade residual flow and leptomeningeal collateral flow[J]. Journal of Cerebral Blood Flow & Metabolism, 2020, 40(1): 126-134. |
| [11] |
GOLDSWEIG A M, SECEMSKY E A. Vascular access and closure for peripheral arterial intervention[J]. Interventional Cardiology Clinics, 2020, 9(2): 117-124. DOI:10.1016/j.iccl.2019.11.001 |
| [12] |
GAO B L, XU G Q, WANG Z L, et al. Transradial stenting for carotid stenosis in patients with bovine type and type Ⅲ aortic arch: experience in 28 patients[J]. World Neurosurgery, 2018, 111: e661-e667. DOI:10.1016/j.wneu.2017.12.138 |
| [13] |
KOLKAILAH A A, ALRESHQ R S, MUHAMMED A M, et al. Transradial versus transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease[J]. Cochrane Database of Systematic Reviews, 2018(4): CD012318. |
| [14] |
CASANA R, BISSACCO D, MALLOGGI C, et al. Aortic arch types and postoperative outcomes after carotid artery stenting in asymptomatic and symptomatic patients[J]. International Angiology, 2020, 39(6): 485-491. |
| [15] |
SHEN S H, JIANG X J, DONG H, et al. Effect of aortic arch type on technical indicators in patients undergoing carotid artery stenting[J]. Journal of International Medical Research, 2019, 47(2): 682-688. DOI:10.1177/0300060518807604 |
| [16] |
MARROCCO-TRISCHITTA M M, VITALE R, NAVA G, et al. Poor concordance between definitions of type Ⅲ arch and implications for risk prediction and assessment for carotid artery stenting[J]. Journal of Vascular Surgery, 2021, 73(4): 1277-1281. DOI:10.1016/j.jvs.2020.08.142 |
| [17] |
MARROCCO-TRISCHITTA M M, BARONI I, VITALE R, et al. Type Ⅲ arch configuration as a risk factor for carotid artery stenting: a systematic review of contemporary guidelines on management of carotid artery stenosis[J]. Annals of Vascular Surgery, 2020, 68: 505-509. DOI:10.1016/j.avsg.2020.04.019 |
| [18] |
DEORA S. Transradial versus transfemoral approach in STEMI: choice is with the operator[J]. Indian Heart Journal, 2020, 72(4): 327-328. DOI:10.1016/j.ihj.2020.06.006 |
| [19] |
AOUN J, HATTAR L, DGAYLI K, et al. Update on complications and their management during transradial cardiac catheterization[J]. Expert Review of Cardiovascular Therapy, 2019, 17(10): 741-751. DOI:10.1080/14779072.2019.1675510 |
| [20] |
GOLDMAN D T, BAGEAC D, MILLS A, et al. Transradial approach for neuroendovascular procedures: a single-center review of safety and feasibility[J]. American Journal of Neuroradiology, 2021, 42(2): 313-318. DOI:10.3174/ajnr.A6971 |
| [21] |
LINDNER S M, MCNEELY C A, AMIN A P. The value of transradial: impact on patient satisfaction and health care economics[J]. Interventional Cardiology Clinics, 2020, 9(1): 107-115. DOI:10.1016/j.iccl.2019.08.004 |
| [22] |
JOSHI K C, BEER-FURLAN A, CROWLEY R W, et al. Transradial approach for neurointerventions: a systematic review of the literature[J]. Journal of Neurointerventional Surgery, 2020, 12(9): 886-892. DOI:10.1136/neurintsurg-2019-015764 |
| [23] |
ZALOCAR LAD, DOROSZUK G, GOLAND J. Transradial approach and its variations for neurointerventional procedures: literature review[J]. Surgical Neurology International, 2020, 11(8): 248. |
| [24] |
BRUNET M C, CHEN S H, PETERSON E C. Transradial access for neurointerventions: management of access challenges and complications[J]. Journal of NeuroInterventional Surgery, 2020, 12(1): 82-86. DOI:10.1136/neurintsurg-2019-015145 |



