冠状病毒属于冠状病毒科(Coronaviridae)冠状病毒属(Coronavirus),是一种单股正链RNA病毒,可引起人类和蝙蝠、骆驼、狗等动物的感染,且主要为呼吸道感染[1]。于2019年12月发生并开始大流行的新型冠状病毒被命名为严重急性呼吸综合征冠状病毒2 (Severe Acute Respiratory Syndrome Coronavirus 2,SARS-CoV-2),又称为2019年新型冠状病毒(2019-nCoV),该病毒导致新型冠状病毒感染(Coronavirus Disease-2019,COVID-19)的发生。COVID-19传播的方式包括动物与人、人与人之间的传播(通过气溶胶传播、院内相关感染传播和母体传播)[2]。新型冠状病毒感染动物后可引起多种疾病,人类受感染后所表现出的症状也因人而异,可能从无症状到危重症,常表现为发热、咳嗽、呼吸困难、胃肠道症状等,严重时可导致严重的肺损伤,需要住院治疗,甚至还可能导致死亡[3]。重症COVID-19患者定义为SARS-CoV-2感染者出现严重缺氧状态,SpO2 < 94%,PaO2/FiO2 < 300 mmHg,呼吸频率>30次/分,或肺部浸润>50%[4]。男性、高龄和合并高血压、糖尿病、心血管疾病、恶性肿瘤等基础疾病会使重症新型冠状病毒感染的风险增加[5]。
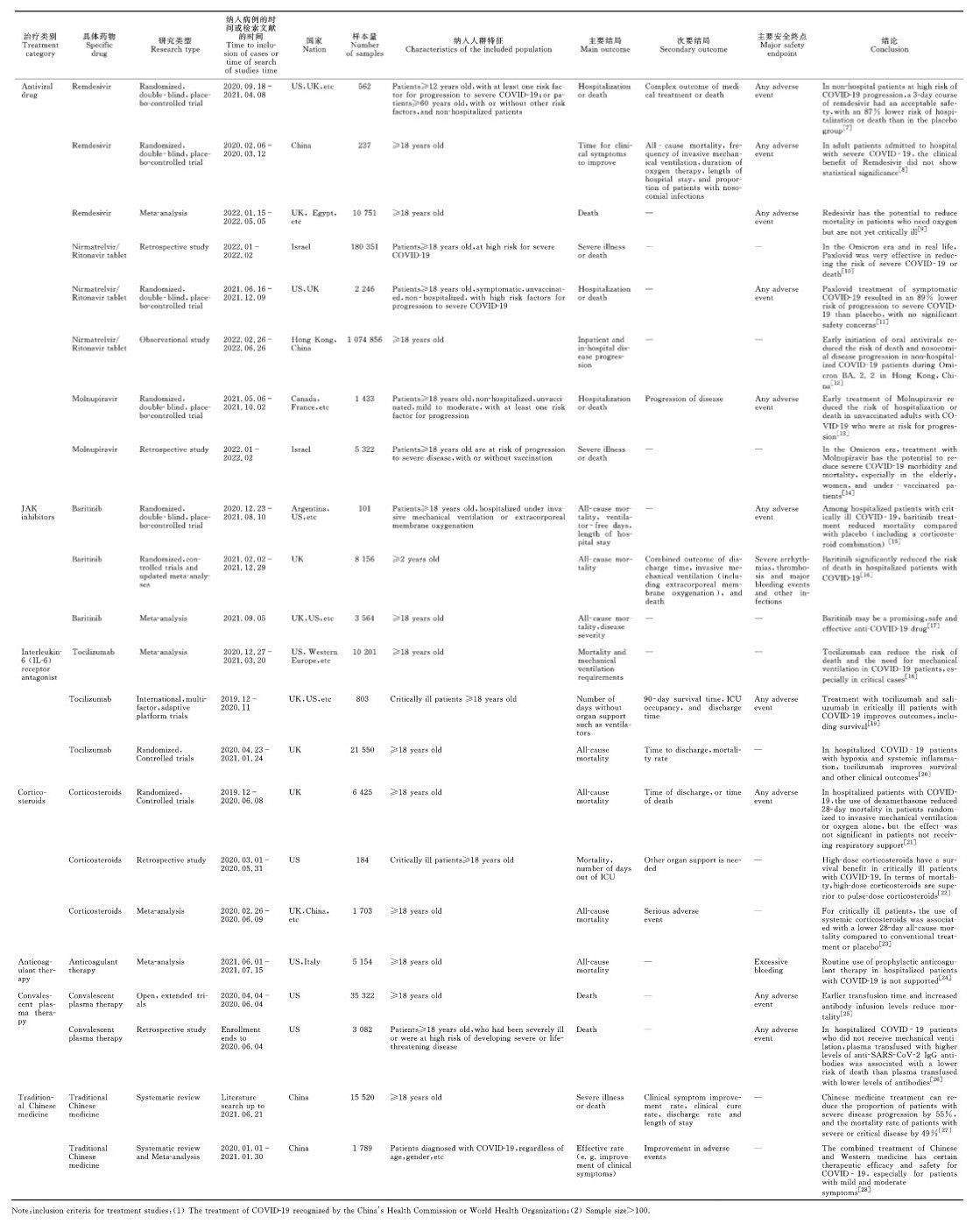
世界卫生组织(World Health Organization,WHO)最新数据显示,截至2023年5月24日,全球累计报告确诊COVID-19病例766 895 075例,死亡6 935 889例,其中我国确诊病例99 261 812例,死亡121 144例[6],新型冠状病毒感染疫情给全球人口及经济带来了巨大的损失。如何有效预防及治疗重症COVID-19患者是一个重大挑战。世界各国在新冠疫情发生后,对新型冠状病毒感染的预防及治疗药物进行了系列研发,现将部分治疗药物研究情况列于表 1。
1 抗病毒药物
抗病毒是病毒感染的特异性治疗,我国目前关于COVID-19的抗病毒药物已经上市的包括3种国产口服药[阿兹夫定、氢溴酸氘瑞米德韦(VV-116)和先诺特韦片/利托那韦片]和2种进口口服药[奈玛特韦/利托那韦(Paxlovid)和莫诺拉韦(Molnupiravir)]。
阿兹夫定、VV-116和Molnupiravir的作用靶点均为依赖RNA的RNA聚合酶(RdRp),可在宿主细胞中通过抑制RdRp的活性,阻断RNA的合成和复制。阿兹夫定、VV-116均用于治疗轻、中症COVID-19成年患者,但有临床数据显示阿兹夫定也能显著缩短中、重症患者的核酸转阴时间及治疗时间。Najjar-Debbiny等[29]的研究结果表明,Molnupiravir可降低进展为严重COVID-19的风险和死亡率。瑞德西韦作用靶点也为RdRp,一项针对非住院COVID-19患者的临床试验表明,瑞德西韦治疗3 d后的患者住院或死亡风险降低了87%[30]。部分随机对照试验、Meta分析结果表明瑞德西韦可降低伴有进展高风险的COVID-19患者的死亡率[31]。WHO推荐对于重症、危重症患者静脉注射使用瑞德西韦。
先诺特韦片/利托那韦片和Paxlovid的作用靶点为冠状病毒主蛋白酶(即3CL蛋白酶),先诺特韦片/利托那韦片是首款国产的抗3CL蛋白酶的抗病毒创新药物,临床研究显示它可有效缩短症状缓解时间及核酸转阴时间;同时可显著降低病毒载量,给药5 d后的病毒载量下降96.28%[32]。但先诺特韦片/利托那韦片尚缺乏降低重症以及死亡风险方面的研究。Paxlovid可用于治疗重症COVID-19患者,临床研究数据显示该药可将COVID-19患者的住院或死亡风险降低89%[33]。Paxlovid也存在局限性,其对高龄、具有基础疾病等特征的高危群体有效,而对于没有基础疾病或感染超过5 d的高危群体治疗效果可能相对较弱。
此外,其他抗病毒药物例如洛匹那韦-利托那韦片(Kaletra)和达芦那韦比司他(Prezcobix)为蛋白酶抑制剂,是治疗艾滋病的药物,也有研究验证其对COVID-19患者的治疗效果。一项小型回顾性研究发现在重症COVID-19患者中使用Kaletra并不能明显改善预后[34],另一项小型单中心临床试验将轻症患者分别给予Prezcobix联合干扰素α-2b治疗或单用干扰素α-2b治疗,结果显示Prezcobix未能加速病毒的清除[35]。法匹拉韦(Favipiravir)是一种广谱抗RNA病毒药物,是我国第一个批准上市的、对COVID-19具有潜在疗效的药物。一项随机对照研究将患者随机分组给予氯喹和法匹拉韦治疗,结果显示法匹拉韦可减少患者住院时间和机械通气需求[36]。但这些抗病毒药物由于有效性不足,目前的研究纳入病例数较少或仅针对轻、中症患者有效,限制了其在COVID-19患者中的使用,目前我国的治疗指南均未将这些药物纳入其中。
2 Janus激酶抑制剂在重症COVID-19患者中,宿主免疫反应在驱动急性炎症过程中起着关键作用。包括白细胞介素-6 (IL-6)在内的几种细胞因子在重症COVID-19患者中含量水平升高,Janus激酶(JAK)抑制剂则可抑制细胞因子风暴,并且通过阻止AP-2相关蛋白激酶1(AAK1)介导的内吞作用来抑制病毒组装。因此,JAK抑制剂被建议用作重症COVID-19患者的治疗药物[37]。巴瑞替尼(Baricitinib)是一种JAK1/JAK2抑制剂,具有抗细胞因子及抗病毒活性。Patoulias等[38]研究表明,在住院COVID-19患者中使用JAK抑制剂(包括巴瑞替尼)治疗可降低43%的死亡风险,以及36%的机械通气或体外膜肺氧合风险。部分随机对照试验、Meta分析表明巴瑞替尼可降低死亡率、重症监护病房的入院率以及对有创机械通气的需求[39, 40]。上述研究结果提示,巴瑞替尼是一种有希望、有前景、安全有效的抗重症COVID-19药物,且生产及储存方便,但需要提供更多的数据来支持其在更广泛人群中的使用。WHO强烈推荐重症、危重症患者使用皮质类固醇、IL-6受体拮抗剂或者巴瑞替尼治疗,三者还可以联合使用。但由于有效性不够理想且价格不低,巴瑞替尼的使用受到了限制。
3 IL-6受体拮抗剂紧急感染或组织损伤诱导IL-6快速产生,并通过增强包括C反应蛋白、纤维蛋白原和血小板生成素等在内的急性期蛋白和免疫反应来激活宿主防御。但IL-6过度产生可导致过多的IL-6受体信号传导,从而导致免疫反应性疾病[41]。IL-6直接影响血管内皮细胞,而血管内皮细胞可产生几种类型的细胞因子和趋化因子并激活凝血级联反应。以凝血异常和血管渗漏为特征的内皮细胞失调是细胞因子风暴的常见并发症[42]。托珠单抗(Tocilizumab)是一种阻断IL-6受体的单克隆抗体,已被批准用于接受全身性皮质类固醇治疗且需要辅助供氧、机械通气或体外膜肺氧合的住院COVID-19成年患者[43]。针对托珠单抗治疗重症COVID-19患者的部分随机对照试验结果表明,使用托珠单抗治疗重症COVID-19患者的死亡率有所降低[44],但有几项试验结果并未显示出明显的临床获益[45, 46],可能与病例数不足有关,需要更多的回顾性或前瞻性研究来验证其对重症COVID-19患者的治疗效果。WHO推荐重症、危重症患者可使用IL-6受体拮抗剂(托珠单抗)治疗。
4 皮质类固醇新型冠状病毒可能会诱导过度的免疫应答,导致促炎介质过量产生,从而导致急性呼吸窘迫综合征(ARDS)、弥散性血管内凝血和多器官衰竭。因此,减轻COVID-19引起的过激炎症也至关重要[47]。皮质类固醇具有强大的抗炎作用,是治疗COVID-19引起的过激炎症的理想药物。Karakoc等[48]进行了一项大剂量皮质类固醇冲击治疗重症COVID-19患者的回顾性研究,结果显示在给予大剂量皮质类固醇后,77.8%重症患者的实验室指标及临床症状均有所改善。Sterne等[49]所做的Meta分析结果显示,对危重COVID-19患者给予全身性皮质类固醇治疗,可降低28天全因死亡率。但关于全身性皮质类固醇治疗重症COVID-19患者最有效的类型、剂量或时机的证据仍不成熟,缺乏多中心、样本量充足的大型研究,且对儿童的影响尚不清楚。美国国立卫生研究院(National Institutes of Health,NIH)所发布的指南建议,对于重症患者的治疗为每日给予1次地塞米松治疗,剂量为6 mg,持续10 d,这也是目前美国医院治疗重症COVID-19患者的方案[50]。WHO也强烈推荐使用皮质类固醇治疗重症、危重症COVID-19患者。
5 抗凝药物COVID-19可能与凝血功能异常有关,包括D-二聚体、凝血酶原时间、部分凝血活酶时间等指标[51]。Zhou等[52]研究报道COVID-19患者相关凝血功能障碍与不良预后(包括死亡率、重症监护病房入住率、需要通气支持)相关。COVID-19患者可能因过激炎症反应、内皮功能障碍和血流淤滞而易发生血栓并发症。由于COVID-19住院患者(尤其是重症患者)发生静脉血栓风险增加,目前推荐使用低分子肝素或普通肝素进行血栓预防[53]。一项对照试验显示,给予治疗剂量的抗凝治疗可显著增加无器官支持天数,增加生存的可能性[54]。一项纳入11项随机临床试验和17项观察性研究的Meta分析结果表明,COVID-19患者的治疗性抗凝治疗和预防性抗凝治疗的死亡率并无显著差异;对于非危重症COVID-19患者,预防性抗凝治疗优于治疗性抗凝治疗;而观察性研究分析结果表明,危重症COVID-19患者进行治疗性抗凝治疗是必要的[55]。NIH指南建议对于无治疗性抗凝指征的患者,除非有禁忌证,均应给予预防性抗凝治疗,且预防性剂量的肝素也适用于孕妇;对于在非ICU病房中开始服用治疗剂量的肝素然后转到ICU病房的患者,建议改用预防性剂量的肝素。由于血栓并发症可能会增加COVID-19患者的死亡风险,预防性或治疗性抗凝至关重要,住院患者排除禁忌证后应给予普通肝素或低分子肝素抗凝治疗,并且与COVID-19相关的静脉血栓栓塞应抗凝治疗至少3个月。
6 恢复期血浆恢复期血浆是中和抗体的来源,由于其含有足够浓度的中和抗体,在早期疾病治疗时效果最好[56]。恢复期血浆治疗是指采集COVID-19痊愈患者的血浆成分,输注给目前重症COVID-19患者,对危重症患者有一定疗效。Joyner等[25]研究分析了美国2 807家急症护理机构的住院患者使用恢复期血浆的情况,结果显示在诊断COVID-19后3 d内输血的患者的7天死亡率为8.7%,在诊断后4 d或更长时间内输血的患者的7天死亡率为11.9%,30天死亡率的结果类似(21.6% vs 26.7%);对于接受高IgG、中等IgG、低IgG血浆的患者,其7天死亡率分别为8.9%、11.6%、13.7%,而且30天死亡率的结果也类似。这些结果表明,早期输注高抗体水平恢复期血浆可降低重症患者的死亡率。Joyner等[57]的一项回顾性分析表明,在未接受机械通气的COVID-19住院患者中,输注较高IgG抗体水平的血浆比输注抗体水平较低的血浆的死亡风险更低。但Alemany等[58]进行的一项研究报道认为,恢复期血浆未能防止轻症COVID-19患者进展为重症,也未能有效降低门诊患者的病毒载量。综上,目前恢复期血浆治疗COVID-19患者的有效性证据不足。虽然美国食品药品监督管理局(Food and Drug Administration, FDA)已授权使用康复期患者的血浆来治疗重症患者,但是目前美国对院内患者不常规使用,对免疫抑制患者(例如器官抑制患者)才使用恢复期血浆。WHO均不推荐普通型、重型患者使用恢复期血浆。我国《新型冠状病毒感染诊疗方案(试行第十版)》指出, 恢复期血浆可在病程早期用于伴有重症高风险、病毒载量高、病情进展快的患者。
7 中药目前有关中药治疗对COVID-19的有效性也有深入研究,多项临床研究表明,中西医联合治疗可以提高COVID-19患者的治愈率,缩短平均住院时间[59]。中药联合常规治疗对重症患者有保护作用,清肺排毒汤联合西医常规治疗的研究表明该治疗措施可改善重症患者的症状[60]。中药治疗重症COVID-19患者的可能机制有:(1)直接抑制病毒——富含类黄酮化合物的中药具有抗病毒作用;(2)抑制炎症,调节免疫功能;(3)抑制血栓形成,抑制血小板的黏附和聚集;(4)抑制氧化应激反应,例如甘草酸可以抗氧化应激,且能抑制炎症反应[61, 62]。我国《新型冠状病毒感染诊疗方案(试行第十版)》推荐,参照指南合理使用中药辅助治疗COVID-19患者。
8 正在申请上市或正在研究的药物普克鲁胺是一种雄激素受体拮抗剂,可以下调血管紧张素转化酶2 (ACE2)和跨膜丝氨酸蛋白酶2 (TM-PRSS2)的活性,这两种酶是SARS-CoV-2侵入宿主细胞的关键蛋白,因此普克鲁胺可以抑制病毒进入宿主细胞,目前已在巴拉圭获批用于紧急治疗COVID-19患者。2021年12月,美国治疗轻、中症非住院COVID-19患者的Ⅲ期临床试验中期数据显示,普克鲁胺的有效性无统计学意义[63]。2022年4月,普克鲁胺的全球多中心Ⅲ期临床试验数据显示,普克鲁胺可有效降低COVID-19患者的住院或死亡率,尤其是用药超过7 d的患者,普克鲁胺的保护率可达100%[64]。目前苏州开拓药业股份有限公司已就该药物向我国国家药品监督管理局申请上市,尚在审评中。
度维利塞胶囊(Duvelisib)是一种磷脂酰肌醇-3激酶(PI3K)的小分子抑制剂,是多种肿瘤的口服治疗药物。Duvelisib可能会通过抑制PI3K来抑制先天性免疫系统的异常过度激活,优先极化巨噬细胞,减少肺部炎症,并减弱病毒持久性,从而改善患者的预后。一项研究探讨了Duvelisib对重症COVID-19患者的作用,共纳入28名重症成年患者,结果显示使用Duvelisib治疗组患者的6个月内的全因死亡率为40%,而使用安慰剂组为61.54%。
Ensitrelvir (S-217622)作用靶点是3CL蛋白酶,作用机制与Paxlovid相同。2022年2月,日本盐野义制药公布S-217622的Ⅱ/Ⅲ期临床试验部分数据,结果显示S-217622组病毒滴度和病毒RNA迅速下降[65]。此外,研究者正计划向中、美两国递交3CL蛋白酶抑制剂ASC11和RdRp抑制剂ASC10的临床研究申请,还有上海君实生物医药科技股份有限公司的3CL蛋白酶抑制剂VV993、广东众生药业股份有限公司的3CL蛋白酶抑制剂RAY003,以及科兴生物制药股份有限公司的RdRp抑制剂SHEN26等多种治疗药物处于临床前研发阶段[66]。这些正在申请上市或正在研究中的药物同时也需要进一步多中心和大样本量数据来证实它们在COVID-19治疗中的有效性和安全性。
9 药物开发治疗COVID-19患者的其他药物正在开发,例如通过多靶点防止病毒进入宿主细胞或通过抑制主要蛋白酶来抑制复制和转录复合物的形成。研究报道靶向S蛋白肽可阻止病毒进入宿主细胞,如Curreli等[67]构建的吻合肽NYBSP-4作用靶向于S1刺突蛋白中的受体结合域,但未在体内模型进行研究;Karoyan等[68]设计的人血管紧张素转换酶2肽模拟物的3种肽(P8、P9和P10),但也未进行体内研究。Bestle等[69]研究表明,TMPRSS2肽模拟抑制剂MI-432、MI-1900均可抑制SARS-CoV-2复制。另外,与靶向S蛋白肽的S1/S2切割位点结合并阻止宿主蛋白酶分解的治疗药物还需进一步研究。抑制病毒进入宿主细胞和抑制复制、转录复合物形成的治疗策略正在开发并不断取得进展。
在重症COVID-19患者中,高炎症综合征和细胞因子风暴与不良预后相关。对患者已使用抗炎药和免疫调节剂,但未能成功规避重症患者的免疫应答加剧,可能是由于细胞因子相互作用的复杂性以及炎症途径的多样性,使得药物对其中一个或几个分子的抑制不足,难以逆转炎症风暴;也可能是这些药物存在生物利用度差、稳定性差和药代动力学不良的缘故[70]。因此,开发合适的药物输送系统也至关重要。药物输送系统可用于承载常规抗炎药或免疫调节剂等,特别是生物利用度差和不稳定的药物,可安全有效地将药物输送到治疗靶点。
10 展望目前COVID-19仍然存在。重症COVID-19患者病死率高,目前暂无特效药,接种疫苗仍是最有效的疫情防控手段,可有效降低重症发生率和死亡率。感染SARS-CoV-2后,病程的第1阶段是病毒复制期,因此抑制病毒复制的治疗在疾病早期更有效,应尽早使用抗病毒治疗。病程的第2、3阶段处于免疫因子风暴期,此时抗病毒治疗已作用不大,主要为抗炎、抑制免疫治疗。临床医生必须综合患者的临床表现及辅助检查来判断疾病的严重程度,这对治疗药物的选择有重要意义。NIH建立了“加速COVID-19治疗干预和疫苗(ACTIV)”联盟,旨在开发COVID-19治疗药物[71]。目前关于多种单克隆抗体、免疫调节剂、抗凝剂和其他治疗药物的临床研究正在ACTIV联盟内进行,大多数药物现在都处于Ⅲ期试验中,相信在不久的将来会有更多更安全有效的治疗COVID-19的药物面市。
| [1] |
DE WIT E, VAN DOREMALEN N, FALZARANO D, et al. SARS and MERS: recent insights into emerging coronaviruses[J]. Nature Reviews Microbiology, 2016, 14(8): 523-534. DOI:10.1038/nrmicro.2016.81 |
| [2] |
SHARMA A, FAROUK I A, LAL S K. COVID-19:a review on the novel coronavirus disease evolution, transmission, detection, control and prevention[J]. Viruses, 2021, 13(2): 202. DOI:10.3390/v13020202 |
| [3] |
FEHR A R, PERLMAN S. Coronaviruses: an overview of their replication and pathogenesis[J]. Methods in Molecular Biology, 2015, 1282: 1-23. |
| [4] |
ATTAWAY A H, SCHERAGA R G, BHIMRAJ A, et al. Severe covid-19 pneumonia: pathogenesis and clinical management[J]. BMJ, 2021, 372(436): 1-19. |
| [5] |
GRASSELLI G, ZANGRILLO A, ZANELLA A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy[J]. JAMA, 2020, 323(16): 1574-1581. DOI:10.1001/jama.2020.5394 |
| [6] |
World Health Organization. WHO coronavirus (COVID-19) dashboard[EB/OL]. (2023-05-24)[2023-05-24]. https://covid19.who.int.
|
| [7] |
GOTTLIEB R L, VACA C E, PAREDES R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients[J]. New England Journal of Medicine, 2022, 386(4): 305-315. DOI:10.1056/NEJMoa2116846 |
| [8] |
WANG Y M, ZHANG D Y, DU G H, et al. Remdesivir in adults with severe COVID-19:a randomised, double-blind, placebo-controlled, multicentre trial[J]. The Lancet, 2020, 395(10236): 1569-1578. DOI:10.1016/S0140-6736(20)31022-9 |
| [9] |
LEE T C, MURTHY S, DEL CORPO O, et al. Remdesivir for the treatment of COVID-19:a systematic review and meta-analysis[J]. Clinical Microbiology and Infection, 2022, 28(9): 1203-1210. DOI:10.1016/j.cmi.2022.04.018 |
| [10] |
NAJJAR-DEBBINY R, GRONICH N, WEBER G, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients[J]. Clinical Infectious Diseases, 2023, 76(3): e342-e349. DOI:10.1093/cid/ciac443 |
| [11] |
HAMMOND J, LEISTER-TEBBE H, GARDNER A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19[J]. New England Journal of Medicine, 2022, 386(15): 1397-1408. DOI:10.1056/NEJMoa2118542 |
| [12] |
WONG C K H, AU I C H, LAU K T K, et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study[J]. The Lancet, 2022, 400(10359): 1213-1222. DOI:10.1016/S0140-6736(22)01586-0 |
| [13] |
BERNAL A J, DA SILVA G M M, MUSUNGAIE D B, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients[J]. New England Journal of Medicine, 2022, 386(6): 509-520. DOI:10.1056/NEJMoa2116044 |
| [14] |
NAJJAR-DEBBINY R, GRONICH N, WEBER G, et al. Effectiveness of molnupiravir in high-risk patients: a propensity score matched analysis[J]. Clinical Infectious Diseases, 2023, 76(3): 453-460. DOI:10.1093/cid/ciac781 |
| [15] |
ELY E W, RAMANAN A V, KARTMAN C E, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial[J]. The Lancet Respiratory Medicine, 2022, 10(4): 327-336. DOI:10.1016/S2213-2600(22)00006-6 |
| [16] |
ABANI O, ABBAS A, ABBAS F, et al. Baricitinib in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial and updated meta-analysis[J]. The Lancet, 2022, 400(10349): 359-368. DOI:10.1016/S0140-6736(22)01109-6 |
| [17] |
LIN Z W, NIU J Y, XU Y F, et al. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis[J]. Journal of Medical Virology, 2022, 94(4): 1523-1534. DOI:10.1002/jmv.27482 |
| [18] |
WEI Q, LIN H, WEI R G, et al. Tocilizumab treatment for COVID-19 patients: a systematic review and meta-analysis[J]. Infectious Diseases of Poverty, 2021, 10(1): 71. DOI:10.1186/s40249-021-00857-w |
| [19] |
BROWN M J, ALAZAWI W, KANONI S. Interleukin-6 receptor antagonists in critically ill patients with Covid-19[J]. New England Journal of Medicine, 2021, 384(16): 1491-1502. DOI:10.1056/NEJMoa2100433 |
| [20] |
ABANI O, ABBAS A, ABBAS F, et al. Tocilizumab in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial[J]. The Lancet, 2021, 397(10285): 1637-1645. DOI:10.1016/S0140-6736(21)00676-0 |
| [21] |
The Recovery Collaborative Group. Dexamethasone in hospitalized patients with Covid-19[J]. New England Journal of Medicine, 2021, 384(8): 693-704. DOI:10.1056/NEJMoa2021436 |
| [22] |
YAQOOB H, GREENBERG D, HWANG F, et al. Comparison of pulse-dose and high-dose corticosteroids with no corticosteroid treatment for COVID-19 pneumonia in the intensive care unit[J]. Journal of Medical Virology, 2022, 94(1): 349-356. DOI:10.1002/jmv.27351 |
| [23] |
STERNE J A C, MURTHY S, DIAZ J V, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19:a meta-analysis[J]. JAMA, 2020, 324(13): 1330-1341. DOI:10.1001/jama.2020.17023 |
| [24] |
ORTEGA-PAZ L, GALLI M, CAPODANNO D, et al. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19:a systematic review and meta-analysis of randomized controlled trials[J]. European Heart Journal: Cardiovascular Pharmacotherapy, 2022, 8(7): 677-686. DOI:10.1093/ehjcvp/pvab070 |
| [25] |
JOYNER M J, SENEFELD J W, KLASSEN S A, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience[Z/OL]. (2020-08-12)[2023-05-24]. https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1.full.pdf.
|
| [26] |
JOYNER M J, CARTER R E, SENEFELD J W, et al. Convalescent plasma antibody levels and the risk of death from Covid-19[J]. New England Journal of Medicine, 2021, 384(11): 1015-1027. DOI:10.1056/NEJMoa2031893 |
| [27] |
KANG X M, JIN D, JIANG L L, et al. Efficacy and mechanisms of traditional Chinese medicine for COVID-19:a systematic review[J]. Chinese Medicine, 2022, 17(1): 30. DOI:10.1186/s13020-022-00587-7 |
| [28] |
LI L, XIE H L, WANG L, et al. The efficacy and safety of combined chinese herbal medicine and western medicine therapy for COVID-19:a systematic review and meta-analysis[J]. Chinese Medicine, 2022, 17(1): 77. DOI:10.1186/s13020-022-00600-z |
| [29] |
NAJJAR-DEBBINY R, GRONICH N, WEBER G, et al. Effectiveness of molnupiravir in high-risk patients: a propensity score matched analysis[J]. Clinical Infectious Diseases, 2023, 76(3): 453-460. DOI:10.1093/cid/ciac781 |
| [30] |
GOTTLIEB R L, VACA C E, PAREDES R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients[J]. New England Journal of Medicine, 2022, 386(4): 305-315. DOI:10.1056/NEJMoa2116846 |
| [31] |
SIEMIENIUK R A C, BARTOSZKO J J, ZERAATKAR D, et al. Drug treatments for Covid-19:living systematic review and network meta-analysis[J]. British Medical Journal, 2020, 370: m2980. |
| [32] |
林志吟. 首款国产3CL新冠药上市高危人群能否获益[N]. 第一财经日报, 2023-03-03(A04).
|
| [33] |
MAHASE E. Covid-19:Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports[J]. British Medical Journal, 2021, 375: n2713. |
| [34] |
王九龙, 卢青, 张波, 等. 重型危重型COVID-19患者洛匹那韦/利托那韦的临床应用及疗效分析[J]. 华南国防医学杂志, 2020, 34(8): 563-568. |
| [35] |
陈军, 夏露, 徐庆年, 等. 达芦那韦/考比司他治疗COVID-19的抗病毒活性和安全性[J]. 上海医药, 2020, 41(S1): 70. |
| [36] |
DABBOUS H M, ABD-ELSALAM S, EL-SAYED M H, et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study[J]. Archives of Virology, 2021, 166(3): 949-954. DOI:10.1007/s00705-021-04956-9 |
| [37] |
RICHARDSON P, GRIFFIN I, TUCKER C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease[J]. The Lancet, 2020, 395: e30-e31. DOI:10.1016/S0140-6736(20)30304-4 |
| [38] |
PATOULIAS D, DOUMAS M, PAPADOPOULOS C, et al. Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials[J]. Clinical Rheumatology, 2021, 40(11): 4671-4674. DOI:10.1007/s10067-021-05884-4 |
| [39] |
ELY E W, RAMANAN A V, KARTMAN C E, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial[J]. The Lancet Respiratory Medicine, 2022, 10(4): 327-336. DOI:10.1016/S2213-2600(22)00006-6 |
| [40] |
SELVARAJ V, FINN A, LAL A, et al. Baricitinib in hospitalised patients with COVID-19:a meta-analysis of randomised controlled trials[J]. EClinicalMedicine, 2022, 49: 101489. DOI:10.1016/j.eclinm.2022.101489 |
| [41] |
KISHIMOTO T, KANG S J. IL-6 revisited: from rheumatoid arthritis to CAR T cell therapy and COVID-19[J]. Annual Review of Immunology, 2022, 40: 323-348. DOI:10.1146/annurev-immunol-101220-023458 |
| [42] |
KANG S J, KISHIMOTO T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms[J]. Experimental and Molecular Medicine, 2021, 53(7): 1116-1123. DOI:10.1038/s12276-021-00649-0 |
| [43] |
World Health Organization. Coronavirus (COVID-19)|Drugs[Z/OL]. (2022-09-12)[2023-05-25]. https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs.
|
| [44] |
REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19[J]. New England Journal of Medicine, 2021, 384(16): 1491-1502. DOI:10.1056/NEJMoa2100433 |
| [45] |
HERMINE O, MARIETTE X, THARAUX P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with covid-19 and moderate or severe pneumonia: a randomized clinical trial[J]. JAMA Internal Medicine, 2021, 181: 32-40. DOI:10.1001/jamainternmed.2020.6820 |
| [46] |
GUPTA S, WANG W, HAYEK S S, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19[J]. JAMA Internal Medicine, 2021, 181(1): 41-51. DOI:10.1001/jamainternmed.2020.6252 |
| [47] |
COPERCHINI F, CHIOVATO L, CROCE L, et al. Cytokine and growth factor reviews the cytokine storm in COVID-19:an overview of the involvement of the chemokine/chemokine-receptor system[J]. Cytokine and Growth Factor Reviews, 2020, 53: 25-32. DOI:10.1016/j.cytogfr.2020.05.003 |
| [48] |
KARAKOC H N, AKSOY A, AYDIN M, et al. Outcome of patients with severe COVID-19 pneumonia treated with high-dose corticosteroid pulse therapy: a retrospective study[J]. Asian Pacific Journal of Tropical Medicine, 2022, 15(4): 161-171. DOI:10.4103/1995-7645.343881 |
| [49] |
STERNE J A C, MURTHY S, DIAZ J V, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19:a meta-analysis[J]. The Journal of the American Medical Association, 2020, 324(13): 1330-1341. DOI:10.1001/jama.2020.17023 |
| [50] |
STAUFFER W M, ALPERN J D, WALKER P F. COVID-19 and dexamethasone: a potential strategy to avoid steroid-related strongyloides hyperinfection[J]. Journal of the American Medical Association, 2020, 324(7): 623-624. DOI:10.1001/jama.2020.13170 |
| [51] |
CONNORS J M, LEVY J H. COVID-19 and its implications for thrombosis and anticoagulation[J]. Blood, 2020, 135: 2033-2040. DOI:10.1182/blood.2020006000 |
| [52] |
ZHOU F, YU T, DU R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study[J]. The Lancet, 2020, 395(10229): 1054-1062. DOI:10.1016/S0140-6736(20)30566-3 |
| [53] |
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance[Z/OL]. (2020-01-12)[2023-05-24]. https://apps.who.int/iris/bitstream/handle/10665/332299/WHO-2019-nCoV-Clinical-2020.1-eng.pdf.
|
| [54] |
The ATTACC, ACTIV-4a, and REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19[J]. New England Journal of Medicine, 2021, 385(9): 790-802. DOI:10.1056/NEJMoa2105911 |
| [55] |
DUO H, LI Y H, SUN Y J, et al. Effect of therapeutic versus prophylactic anticoagulation therapy on clinical outcomes in COVID-19 patients: a systematic review with an updated meta-analysis[J]. Thrombosis Journal, 2022, 20(1): 47. DOI:10.1186/s12959-022-00408-9 |
| [56] |
CASADEVALL A, DADACHOVA E, PIROFSKI L A. Passive antibody therapy for infectious diseases[J]. Nature Reviews Microbiology, 2004, 2: 695-703. DOI:10.1038/nrmicro974 |
| [57] |
JOYNER M J, CARTER R E, SENEFEL J W, et al. Convalescent plasma antibody levels and the risk of death from Covid-19[J]. New England Journal of Medicine, 2021, 384(11): 1015-1027. DOI:10.1056/NEJMoa2031893 |
| [58] |
ALEMANY A, MILLAT-MARTINEZ P, CORBACHO-MONNE M, et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19:a randomised, placebo-controlled trial[J]. The Lancet Respiratory Medicine, 2022, 10(3): 278-288. DOI:10.1016/S2213-2600(21)00545-2 |
| [59] |
LI C, WANG L, REN L. Antiviral mechanisms of candidate chemical medicines and traditional chinese medicines for SARS-CoV-2 infection[J]. Virus Research, 2020, 286: 198073. DOI:10.1016/j.virusres.2020.198073 |
| [60] |
王芳, 郭旸, 焦丽雯, 等. 清肺排毒汤联合西医常规治疗重型新型冠状病毒肺炎50例临床疗效回顾性分析[J]. 中医杂志, 2021, 62(20): 1801-1805. |
| [61] |
LI R F, HOU Y L, HUANG J C, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2)[J]. Pharmacological Research, 2020, 156: 104761. DOI:10.1016/j.phrs.2020.104761 |
| [62] |
JIA S S, LUO H, LIU X K, et al. Dissecting the novel mechanism of reduning injection in treating coronavirus disease 2019 (COVID-19) based on network pharmacology and experimental verification[J]. Journal of Ethnopharmacology, 2021, 273: 113871. DOI:10.1016/j.jep.2021.113871 |
| [63] |
开拓药业. 开拓药业公布普克鲁胺治疗轻中症新冠患者Ⅲ期临床试验中期分析进展[EB/OL]. (2021-12-27)[2022-09-26]. https://www.kintor.com.cn/news/246.html.
|
| [64] |
开拓药业. 开拓药业公布普克鲁胺治疗轻中症非住院新冠患者Ⅲ期全球多中心临床试验关键数据结果[EB/OL]. (2022-04-06)[2022-09-26]. https://www.kintor.com.cn/news/257.html.
|
| [65] |
张杰, 杨琼梁, 李欣, 等. 4种抗新型冠状病毒肺炎(COVID-19)药物在临床应用与分析[J]. 中国临床药理学杂志, 2022, 38(12): 1392-1397. |
| [66] |
张竞文, 许方婧伟, 张云涛. 新型冠状病毒肺炎口服药物莫诺拉韦及其对比分析[J]. 中国新药杂志, 2022, 31(21): 2144-2151. |
| [67] |
CURRELI F, VICTOR S M B, AHMED S, et al. Stapled peptides based on human angiotensin-converting enzyme 2 (ACE2) potently inhibit SARS-CoV-2 infection in vitro[J]. Microbiome, 2020, 11: e02451-20. |
| [68] |
KAROYAN P, VIEILLARD V, GOEZ-MORALES L, et al. Human ACE2 peptide-mimics block SARS-CoV-2 pulmonary cells infection[J]. Communications Biology, 2021, 4: 197. DOI:10.1038/s42003-021-01736-8 |
| [69] |
BESTLE D, HEINDL M R, LIMBURG H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells[J]. Life Science Alliance, 2020, 3: e202000786. DOI:10.26508/lsa.202000786 |
| [70] |
KAMAT S, KUMARI M, JAYABASKARAN C. Nano-engineered tools in the diagnosis, therapeutics, prevention, and mitigation of SARS-CoV-2[J]. Journal of Controlled Release, 2021, 338: 813-836. DOI:10.1016/j.jconrel.2021.08.046 |
| [71] |
COLLINS F S, STOFELS P. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): an unprecedented partnership for unprecedented times[J]. Journal of the American Medical Association, 2020, 323: 2455-2457. DOI:10.1001/jama.2020.8920 |