酵母作为真菌研究中的模式生物,在工业生产中应用十分广泛,如用于生产啤酒、葡萄酒、面包、异源蛋白质、疫苗和高价值代谢物等[1-4]。不同于实验室的培养条件,工业发酵的酵母需要应对的一个重大挑战是发酵环境的变化,如温度、低pH值、高渗和高浓度乙醇等,并在此过程中产生高价值代谢物,因此酵母菌株耐受胁迫的能力对工业生产十分重要[5-7]。在酵母多重胁迫耐受机制的研究中,耐高温是目前国内外发酵行业研究中十分热门且重要的课题。酵母的最优生长及发酵温度通常为28-35℃,适宜在37℃以上生长的酵母为高温酵母[8]。目前工业上常用的发酵菌株为酿酒酵母,其最适生长温度一般不超过37℃。有报道称,某些酿酒酵母菌株可在高达43℃的温度下产生乙醇,但这种耐高温的酿酒酵母菌株资源极其有限,因此人们常从自然界中分离出多株耐高温的非传统酵母菌株,包括耶氏酵母(Yarrowia)、克鲁维酵母(Kluyveromyces)、念珠菌(Candida)和毕赤酵母(Pichia)等,这些酵母菌株在高温下生长良好且能产生有价值的次级代谢产物[9-13]。一个典型的例子是酵母利用木质纤维素原料发酵生产乙醇,该过程需经过高温预处理和糖化步骤,即将木质素分解为微生物发酵可利用的五碳糖或六碳糖,随后酵母在中低温条件下将糖转化为生物乙醇[14, 15]。同步糖化发酵法(Simultaneous Saccharification and Fermentation,SSF)是乙醇生产的首选方法,其优点是水解和发酵可在同一个发酵罐中进行,操作简单、成本低、完成时间短,缺点是糖化和发酵对温度的要求不同[16]。因此,获得具有耐高温特性的酵母是生物乙醇产业中提高工业生产能力、降低生产成本的关键策略。
酵母的耐高温特性是一个复杂的性状,由多基因协同表达、多蛋白共同作用来决定。目前的研究发现,酵母已经进化出复杂而微妙的机制,可通过调节特定的代谢途径以保护机体免受高温损伤,或通过激活和调节特定的耐热相关基因来合成特定的化合物[17, 18]。为鉴定新基因并阐明酵母耐高温的复杂机制,研究者们对酵母耐高温特性进行了深入的研究。本文主要从代谢途径、基因表达、酶活特性和蛋白质相互作用等4个方面对酵母耐高温分子机制进行综述(图 1),为耐高温酵母的筛选、改造及应用提供理论基础,有助于酵母耐热性的合理设计,提高工业生产的效率并降低生产成本。
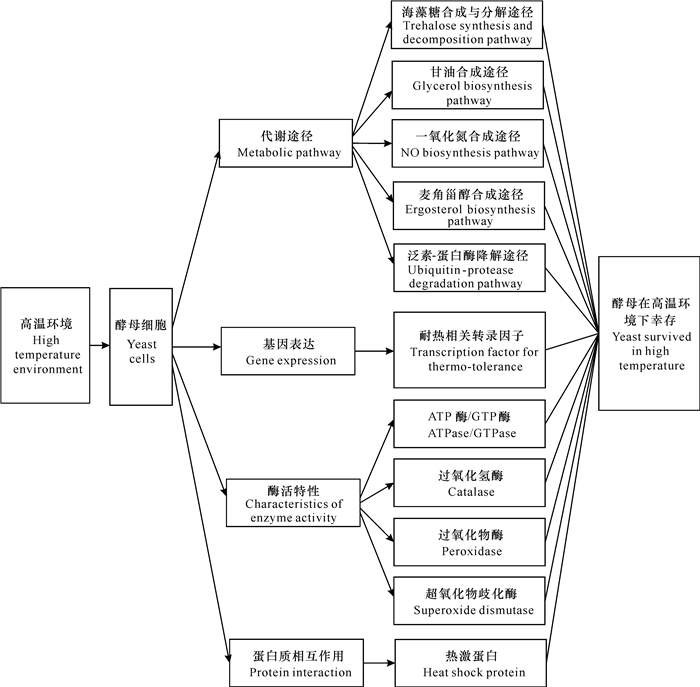
|
| 图 1 酵母耐高温分子机制 Fig. 1 Molecular mechanism of thermo-tolerance in yeast |
1 代谢途径
新陈代谢为细胞发挥功能提供能量和基础,新陈代谢的调节需要对不同的外部信号(如环境变化)和内部信号(如细胞周期状态)作出反应。当酵母处于高温环境下,其代谢途径可根据温度的变化及时调整以适应环境的变化,其中海藻糖合成与分解途径、甘油合成途径、麦角甾醇合成途径、一氧化氮合成途径、泛素-蛋白酶降解途径在应对高温胁迫方面发挥重要的作用[19-22]。
海藻糖是由两分子葡萄糖组成的非还原性双糖,存在于多种生物体中,如细菌、真菌、昆虫和植物等。对称的葡萄糖分子结构具有较好的稳定性,可作为细胞蛋白质和生物膜的应激保护剂以抵抗环境胁迫[23, 24]。在酿酒酵母中,海藻糖由一种酶复合物合成,该酶复合物由tsl1、tps1、tps2和tps3编码的蛋白组成,其中基因tps1和tps2分别编码海藻糖-6-磷酸合成酶和海藻糖-6-磷酸酶,而tps3和tsl1编码复合物的2个调节亚基[25]。海藻糖的分解代谢主要由中性海藻糖酶和酸性海藻糖酶负责[26, 27]。nth1和nth2基因编码的胞浆中性海藻糖酶受cAMP依赖的磷酸化过程、营养物质和温度的调节,负责海藻糖的细胞内稳态或循环;由ath1基因编码的空泡酸性海藻糖酶受营养物质的调控,负责利用胞外海藻糖[28, 29]。当酵母细胞受到高温胁迫时,海藻糖合成与分解途径中参与合成与分解的基因表达水平升高,促进海藻糖的合成与分解,使得细胞既可利用其作为高温保护剂,也可分解为单糖后进入中心糖代谢为细胞应对高温环境供能;当细胞远离胁迫环境时,细胞内海藻糖-6-磷酸含量的增加可抑制Tps1的活性,不利于海藻糖的合成[19, 30]。
甘油是酵母在高温条件下的主要保护剂之一,其主要作用原理是甘油的积累使酵母重新调整细胞膜的渗透压以保证在高温胁迫下细胞功能的正常发挥,从而维持酵母正常的生命活动。在酵母发酵的过程中,甘油是仅次于乙醇和二氧化碳的第三大发酵产物,其合成途径受2个关键基因gpd1和gpd2的调控[31, 32]。有研究证实,gpd1和gpd2基因是细胞在高温胁迫下生长所必需的,且受高渗甘油(High Osmolarity Glycerol,HOG)途径的调节[33, 34]。当酵母受到热刺激时,通过Sho1-Ste20-Ste50-Ste11蛋白级联将信息传递下去可激活HOG途径中关键的蛋白激酶Hog1或通过Nts1将细胞壁完整性(Cell Wall Integrity,CWI)途径与HOG途径连接起来。Hog1由Pbs2磷酸化后进入细胞核激活gpd1和gpd2基因的表达,同时甘油分泌途径中Fsp1通道关闭,导致甘油分泌减少而间接增加细胞内的甘油含量,保护细胞免受高温的侵害[20, 35]。
一氧化氮作为一种信号分子,参与多种细胞功能的调节,其主要来源是精氨酸代谢。当酵母受到高温冲击时,精氨酸可转化为瓜氨酸和一氧化氮,低水平的一氧化氮对酵母具有一定的保护作用[36]。在酵母合成一氧化氮的过程中,除一氧化氮合成酶发挥作用外,酵母的黄素蛋白Tah18也参与其中。基因敲除结果表明,Tah18依赖的一氧化氮合成赋予酵母细胞耐高温胁迫的能力[22]。
麦角甾醇是真菌细胞膜的重要组成部分,决定膜相关蛋白的流动性、通透性和活性[37]。酵母在高温应激下生长可使细胞膜的流动性增加,影响蛋白质折叠和稳定性,破坏细胞骨架结构以及细胞代谢平衡[38]。有研究表明,甾醇脱氢酶Erg3和Erg5的缺失与酵母的耐热性相关,“扁平”甾醇如麦角-8(9), 22-二烯醇或麦角-5, 7, 22, 24(28)-三烯醇替代麦角甾醇不会增加酿酒酵母的耐热性,但“弯曲”甾醇如粪甾醇可增加酵母的耐热性。因此,erg3基因突变后会导致甾醇甲基转移酶Erg6活性增强,Erg6催化酵母甾醇甲基化形成粪甾醇,从而引起“弯曲”甾醇的积累,赋予酵母更高的耐热性[39];erg5基因的缺失,导致细胞积累更多具有饱和侧链的甾醇促使细胞膜具有更好的排序效果,更好地维持细胞膜的流动性,因此可使erg5突变体比未突变erg5的酵母菌株具有更好的耐热性,并且该基因的功能在真菌中具有一定的保守性[21]。此外,有研究表明ABC转运蛋白基因Pdr18的缺失会导致细胞内麦角甾醇合成前体的积累,这种积累可能触发Erg1表达的反馈抑制,使得酵母细胞表现出更高的渗透性以及对多种环境的挑战,如高温环境的敏感性[40]。
高温环境会引起酵母细胞中蛋白的错误折叠从而影响蛋白活性,因此细胞可通过泛素-蛋白酶降解途径降解错误折叠的蛋白以保护细胞免遭功能失调的危机,例如酵母中rsp5基因的过度表达可使细胞具有更高的耐热性[41, 42]。rsp5基因编码E3泛素连接酶,参与蛋白质的泛素化,调节细胞结构中蛋白质的运输和最终降解[43, 44]。此外,Rsp5可与Bul1、Bul2、泛素结合蛋白Ubc1、泛素蛋白酶Ubp4和Ubp16等蛋白相互作用以提高应激反应基因spi1的表达水平,进而提高细胞对热休克和氧化应激的耐受能力[45]。
此外,通过转录组、蛋白质组等技术,已有对高温酵母整体代谢变化趋势的相关研究。例如,通过对定量蛋白质组学和转录组学的数据进行综合分析,Li等[46]发现,酵母高温发酵后,马克斯克鲁维酵母参与转录、翻译、氧化磷酸化和脂肪酸代谢的蛋白下调,一些分子伴侣和蛋白酶体蛋白上调,ATP酶活性显著降低,总脂肪酸逐渐积累。Fu等[13]通过转录组分析发现,高温可刺激马克斯克鲁维酵母的线粒体呼吸,抑制三羧酸TCA循环,导致活性氧(Reactive Oxygen Species,ROS)含量增加,并产生更多麦角甾醇来应对乙醇胁迫。Chamnipa等[47]发现,库德氏毕赤酵母RZ8-1在高温42℃乙醇发酵过程中,热休克蛋白基因(ssq1、hsp90)、乙醇脱氢酶基因(adh1、adh2、adh3、adh4) 以及甘油醛-3-磷酸脱氢酶基因(tdh2) 的表达水平升高,表明有些基因的表达不仅限于乙醇发酵途径,也与酵母的耐高温相关。总之,酵母在高温与适温下的代谢调节存在显著差异,细胞可通过控制耐高温相关代谢基因的表达来调节代谢产物的含量,从而有利于酵母在高温下生存。酵母细胞与耐热相关的代谢途径详见表 1。
| 与耐热相关的代谢途径 Metabolic pathways related to thermo-tolerance |
功能 Function |
参考文献 Reference |
| 海藻糖合成与分解途径 Trehalose sythesis and decomposition pathway |
海藻糖可作为细胞的热保护剂,亦可分解为葡萄糖进入中心糖代谢途径 Trehalose can be used as a thermal protective agent and decomposed into glucose into the central glucose metabolic pathway |
[19, 29] |
| 甘油合成途径 Glycerol biosynthesis pathway |
甘油作为细胞的热保护剂 Glycerol as a thermal protective agent for yeast cells |
[20, 35] |
| 一氧化氮合成途径 NO biosynthesis pathway |
一氧化氮作为信号分子传递信息 NO as a signal molecule transmitting information |
[22, 36] |
| 麦角甾醇合成途径 Ergosterol biosynthesis pathway |
麦角甾醇可调节细胞膜的流动性、稳定性及活性 Ergosterol can regulate the fluidity, stability and activity of cell membrane |
[37] |
| 泛素-蛋白酶降解途径 Ubiquitin-protease degradation pathway |
该途径可降解高温产生的错误蛋白,避免由错误蛋白所引起的细胞功能紊乱 This pathway can degrade misfolded proteins and avoid cell dysfunction caused by high temperature |
[41] |
2 基因表达
生物体耐高温是多基因协同表达以赋予细胞在异常温度下存活和生长的特性。通过分子生物学、分子遗传学、生物信息学、组学分析等手段,目前已有许多耐高温相关基因被鉴定,使得对生物体耐高温的遗传基础有了深入的了解。酵母的耐高温特性是酵母在工业发酵中十分重要的特点之一,当遭遇高温胁迫时,酵母通过调控自身的基因表达水平以适应高温环境。
有研究表明,酵母的转录因子Hsf1、Msn2、Msn4、Yap1、Hac1、Rlm1、Cad1在高温环境下可调控多个基因的表达水平,以提高细胞耐高温的能力[48-54]。热休克转录因子Hsf1非常保守,其核心结构包括翼螺旋-转螺旋DNA结合域以及对三聚体形成至关重要的疏水螺旋区域,此外还有两个分别位于N端和C端的反式激活域。正常情况下Hsf1是不活跃的单体或二聚体形式,当酵母受到高温胁迫时可形成三聚体并高度磷酸化,然后与热应激相关基因的启动子nGAAn反向重复序列结合,发挥其转录活性,激活相关基因的转录[55-57]。Msn2和Msn4已被证实是酿酒酵母中两个对温度敏感的转录激活子,其氨基酸序列具有一定的同源性且识别DNA序列的氨基酸残基高度保守[58]。正常条件下Msn2和Msn4蛋白位于细胞质,当细胞处于高温环境则被运输到细胞核,随后蛋白中的锌指结构可直接结合到DNA序列上或通过其他蛋白间接调控约200个基因的表达[59-61]。氧化休克因子Yap1通过共价作用将传感器蛋白Gpx3激活,使其向细胞核移动,上调抗氧化基因的表达,如Yap1可诱导gsh1和gsh2的表达,以便酵母细胞在处于热休克期间合成还原型谷胱甘肽,从而提高细胞的耐受性[62-64]。转录因子Hac1作为一种碱性亮氨酸拉链蛋白,可与未折叠蛋白反应(Unfolded Protein-Response,UPR)元件结合从而调节参与UPR的基因表达[52]。Rlm1作为蛋白激酶C(Protein Kinase C,PKC)途径的1个转录因子,其转录活性被MAP激酶Mpk1通过Ser427和Thr439的磷酸化来调节,进而调控至少25个与细胞壁合成相关基因的表达,用于维持细胞壁的完整性[53]。Cad1作为转录激活剂,参与蛋白质稳定性相关基因的表达调控[54]。除上述转录因子外,Skn7可与Hsf1相互作用共同激活hsp12、hsp26、hsp70、hsp82和hsp104基因以响应温度变化带来的氧化应激[65]。
3 酶活特性细胞需要不同的氧化环境以促进蛋白质折叠和活性,保证生命活动的正常进行。当细胞遇高温、高渗透压等不利的环境条件时,会导致细胞氧化还原功能障碍,影响细胞生长代谢过程,如信号转导,RNA、DNA和蛋白质的合成以及细胞周期调节等,因此细胞可通过ATP酶催化ATP水解产生能量,维持细胞稳态[66, 67]。酵母的质膜H+-ATP酶(Plasma Membrane H+-ATPase,Pma1)由基因pma1编码,属于分布广泛的一类重要的P2型ATP酶[68]。Pma1是产生电化学质子梯度的重要酶,可穿过质膜,为次级溶质运输系统提供能量,维持离子稳态和细胞内pH值,并间接调节细胞代谢[69]。
生物体赖以生存的环境中营养可用性、渗透压平衡、温度和有害物质在不断变化。为抵御这些外部压力,所有需氧生物都配备了广泛的分子伴侣,如氧化还原分子伴侣[70, 71]。酵母胞浆过氧化物酶PrxS可根据环境条件从低分子量(Low Molecular Weight,LMW)形式转变为高分子量(High Molecular Weight,HMW)形式。当其处于LMW形式时,PrxS主要发挥过氧化物酶功能;而处于HMW形式时,PrxS可作为分子伴侣发挥功能,这种过氧化物酶-分子伴侣功能的转换赋予酵母细胞抗应激能力[72, 73]。有研究表明,酵母细胞遭遇热应激后,可诱导Ytp1 (一种GTP酶)可逆聚成HMW形式,这种结构变化使Ytp1失去原有的GTP酶活性,但赋予该蛋白一种新的分子伴侣活性;当细胞从热应激中恢复,Ytp1蛋白会回到LMW形式,发挥其GTP酶功能。Ytp1蛋白在HMW和LMW形式之间的变换,赋予酵母细胞适应不同温度环境的能力[74, 75]。此外,Rho1也是一个具有GTP酶活性且与酵母氧化应激相关的蛋白,已有报道证实Rho1的突变体对温度敏感且会使胞质中ROS积累水平升高,因此Rho1可通过调节包括Ycf1在内的多种下游靶点来保护酵母细胞免受氧化应激带来的损害[76, 77]。
在正常的细胞代谢过程中,活性氧ROS主要通过呼吸和光合电子传递链不断产生[78]。这些高反应性分子可与多种细胞成分发生反应,破坏DNA、蛋白质和脂质[79]。因此,细胞必须有一定的防护机制严格控制ROS的浓度。当酵母细胞遭受热应激后,会导致细胞的氧化损伤,因此酵母细胞也含有超氧化物歧化酶SOD、过氧化氢酶和过氧化物酶Prx等抗氧化系统,以去除细胞中的活性氧[19]。酵母细胞中的超氧化物歧化酶,包括线粒体基质中的锰超氧化物歧化酶SOD2和胞浆/膜间隙中的铜锌超氧化物歧化酶SOD1,可将过氧化氢歧化为水和二氧化碳,保障电子传递链的正常运行,维持细胞稳态[80]。过氧化物酶体过氧化氢酶A(Cat1)和细胞溶质过氧化氢酶T(Ctt1)在细胞受到不同的应激条件(如热应激)时可被诱导表达,因此这两种酶的活性对于保护酵母细胞在高温条件下免遭氧化损伤的侵害至关重要[81]。过氧化物酶可分为4种类型:2-Cys-Prx,1-Cys-Prx,Prx-Q和Ⅱ型Prx (PrxⅡ),这4种类型的过氧化物酶均具有同样的反应机理,即催化半胱氨酸接收来自过氧化氢的氧原子,生成磺酸后,再由不同的分子(如硫氧还蛋白TRX)将其再生为还原形式,从而达到去除过氧化氢的目的。酿酒酵母的过氧化物酶主要是2-Cys-Prx (Tsa1、Tsa2、Ahp1和Dot5)和1-Cys-Prx (Prx1)两种类型,其中Tsa1和Tsa2已被证实与酵母耐热性相关,而Prx1能够以硫氧还蛋白特有的方式去除过氧化氢, 维持线粒体在高温条件下的稳定性[79]。酵母细胞中与耐热相关的酶见表 2。
| 与耐热相关的蛋白酶 Enzymes related to thermo-tolerance |
功能 Function |
参考文献 Reference |
| ATP酶 ATPase |
为细胞提供能量 Provide energy for cells |
[66] |
| GTP酶 GTPase |
为细胞提供能量或作为分子伴侣 Provide energy or act as molecular chaperones for cells |
[72] |
| 过氧化氢酶 Catalase |
清除细胞中ROS,保证细胞免受氧化损伤 Remove ROS in cell to protect the cells from oxidative damage |
[19] |
| 过氧化物酶 Peroxidase |
清除细胞中ROS,保证细胞免受氧化损伤 Remove ROS in cell to protect the cells from oxidative damage |
[81] |
| 超氧化物歧化酶 Superoxide dismutase |
清除细胞中ROS,保证细胞免受氧化损伤 Remove ROS in cell to protect the cells from oxidative damage |
[80] |
4 蛋白质相互作用
细胞体内含有多种多样的蛋白质,蛋白质发挥自身活性作用或与其他蛋白质相互作用构成细胞的生化网络,维持细胞的生命活动。热休克蛋白可作为分子伴侣在酵母热应激期间协助蛋白质折叠以及复性错误蛋白或激活沉默蛋白,以维持细胞的正常功能。有研究表明,酵母热休克转录因子Hsf1的活性抑制依赖于热休克蛋白Hsp70-Ssa1的直接结合,即Hsp70-Ssa1可利用两个暴露于蛋白表面的半胱氨酸残基与Hsf1结合来抑制Hsf1的活性,当细胞缺乏Hsp70-Ssa1时,转录因子Hsf1不能有效失活[82-84]。除Hsp70外,转录因子Hsf1也可与Hsp90相互作用,增加细胞短期的热适应[85]。Hsp90作为酵母细胞中重要的热休克蛋白,有Hsp82和Hsc82两种亚型,除能与转录因子Hsf1相互作用外,其活性和靶蛋白特异性受到其他分子伴侣,如Sti1、Cns1、Cdc37、Sba1、Aha1、Cpr6、Cpr7和Ppt1的调控。例如Sti1和Cns37可促进Hsp70/Hsp90的相互作用,Cdc37可调节Hsp90作为折叠蛋白酶的活性,防止某些蛋白的聚集,而Sba1、Aha1、Cpr6、Cpr7和Ppt1可控制Hsp90作为ATP酶活性的分子伴侣来调控细胞的能量供应[86-89]。热休克蛋白Hsp104在进化上是Hsp100家族中非常保守的一员,作为一种应激诱导型分子伴侣,可形成六聚体与Hsp70、Hsp40协同作用激活一些损伤蛋白,从而保护酵母细胞免受高温环境胁迫引起的损伤[90]。除上述的热休克蛋白Hsp70和Hsp90外,小热休克蛋白sHsp26通常以二十四聚体的形式存在,当细胞遭受高温时可及时解聚成二聚体与Hsp70、Hsp104相互作用对相关底物进行复性[91, 92]。上述研究表明,酵母可利用不同的热休克蛋白Hsp、小热休克蛋白sHsp、热休克转录因子等蛋白之间的相互作用,提高细胞对温度变化的适应能力,从而促进细胞在高温环境下的生存能力。
5 结束语酵母作为工业上应用广泛的微生物之一,其耐高温的性能对工业发酵具有十分重要的作用。尽管人们对酵母耐高温的分子机制研究已取得较大的进展,但细胞耐受高温胁迫是一个复杂的过程,仍存在许多未解之谜亟待研究,如酵母细胞对高温环境感知与应答的分子机制,是否有其他未报道的转录因子参与耐高温的调控,非传统酵母与传统酵母的耐高温分子机制存在哪些差异,非传统酵母在耐高温胁迫方面优于传统酵母的原因等。因此,对酵母耐高温分子机制进行深入研究,不仅有利于我国发酵工业对耐高温菌株的选育,而且有利于能耗的降低和水资源的利用,简化和优化发酵过程,促进我国发酵工业的环境友好型转变。
| [1] |
GIAEVER G, NISLOW C. The yeast deletion collection: A decade of functional genomics[J]. Genetics, 2014, 197(2): 451-465. DOI:10.1534/genetics.114.161620 |
| [2] |
RAHMAT E, KANG Y. Yeast metabolic engineering for the production of pharmaceutically important secondary metabolites[J]. Applied Microbiology and Biotechnology, 2020, 104(11): 4659-4674. DOI:10.1007/s00253-020-10587-y |
| [3] |
MOKDAD G R, ABDELMOULA S S, HADIJI A N, et al. Yeasts as a tool for heterologous gene expression[J]. Methods in Molecular Biology, 2012, 824: 359-370. |
| [4] |
WALKER G M, WALKER R S K. Enhancing yeast alcoholic fermentations[J]. Advances in Applied Microbiology, 2018, 105: 87-129. |
| [5] |
GARCÍA R E, GUILLAMÓN J M. Mechanisms of yeast adaptation to wine fermentations[J]. Progress in Molecular and Subcellular Biology, 2019, 58: 37-59. |
| [6] |
GOBERT A, TOURDOT M R, SPARROW C, et al. Influence of nitrogen status in wine alcoholic fermentation[J]. Food Microbiology, 2019, 83: 71-85. DOI:10.1016/j.fm.2019.04.008 |
| [7] |
PRETORIUS I S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking[J]. Yeast, 2000, 16(8): 675-729. DOI:10.1002/1097-0061(20000615)16:8<675::AID-YEA585>3.0.CO;2-B |
| [8] |
PRADO C D, MANDRUJANO G P L, SOUZA J P, et al. Physiological characterization of a new thermotolerant yeast strain isolated during Brazilian ethanol production, and its application in high-temperature fermentation[J]. Biotechnology for Biofuels, 2020, 13: 178. DOI:10.1186/s13068-020-01817-6 |
| [9] |
LEDESMA-AMARO R, NICAUD J M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids[J]. Prog Lipid Research, 2016, 61: 40-50. DOI:10.1016/j.plipres.2015.12.001 |
| [10] |
KARIM A, GERLIANI N, AÏDER M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology[J]. International Journal of Food Microbiology, 2020, 333: 108818. DOI:10.1016/j.ijfoodmicro.2020.108818 |
| [11] |
CHOUDHARY J, SINGH S, NAIN L. Bioprospecting thermotolerant ethanologenic yeasts for simultaneous saccharification and fermentation from diverse environments[J]. Journal of Bioscience and Bioengineering, 2017, 123(3): 342-346. DOI:10.1016/j.jbiosc.2016.10.007 |
| [12] |
YANG Z L, ZHANG Z S. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: A review[J]. Biotechnology Advances, 2018, 36(1): 182-195. DOI:10.1016/j.biotechadv.2017.11.002 |
| [13] |
FU X F, LI P S, ZHANG L, et al. Understanding the stress responses of Kluyveromyces marxianus after an arrest during high-temperature ethanol fermentation based on integration of RNA-Seq and metabolite data[J]. Applied Microbiology and Biotechnology, 2019, 103(6): 2715-2729. DOI:10.1007/s00253-019-09637-x |
| [14] |
宁艳春, 陈希海, 王硕, 等. 纤维素乙醇研发现状与研究趋势分析[J]. 化工科技, 2020, 28(1): 65-68. |
| [15] |
曹运齐, 刘云云, 胡南江. 燃料乙醇的发展现状分析及前景展望[J]. 生物技术通报, 2019, 35(4): 163-170. |
| [16] |
IBRAHIM M F, ABD-AZIZ S, YUSOFF M E M, et al. Simultaneous enzymatic saccharification and ABE fermentation using pretreated oil palm empty fruit bunch as substrate to produce butanol and hydrogen as biofuel[J]. Renew Energy, 2015, 77: 447-455. DOI:10.1016/j.renene.2014.12.047 |
| [17] |
MATSUSHITA K, AZUMA Y, KOSAKA T, et al. Genomic analyses of thermotolerant microorganisms used for high-temperature fermentations[J]. Bioscience, Biotechnology and Biochemistry, 2016, 80(4): 655-668. DOI:10.1080/09168451.2015.1104235 |
| [18] |
TAKAGI H. Molecular mechanisms and highly functional development for stress tolerance of the yeast Saccharomyces cerevisiae[J]. Bioscience Biotechnology and Biochemistry, 2021, 85(5): 1017-1037. DOI:10.1093/bbb/zbab022 |
| [19] |
GAO L M, LIU Y Q, SUN H, et al. Advances in mechanisms and modifications for rendering yeast thermotolerance[J]. Journal of Bioscience and Bioengineering, 2016, 121(6): 599-606. DOI:10.1016/j.jbiosc.2015.11.002 |
| [20] |
GANG L, KIWON S. Direct interaction of Ste11 and Mkk1/2 through Nst1 integrates high-osmolarity glycerol and pheromone pathways to the cell wall integrity MAPK pathway[J]. FEBS Letters, 2016, 590(1): 148-160. DOI:10.1002/1873-3468.12039 |
| [21] |
LIU G D, CHEN Y, FÆRGEMAN N J, et al. Elimination of the last reactions in ergosterol biosynthesis alters the resistance of Saccharomyces cerevisiae to multiple stresses[J]. FEMS Yeast Research, 2017, 17(6): fox063. DOI:10.1093/femsyr/fox063 |
| [22] |
NISHIMURA A, KAWAHARA N, TAKAGI H. The flavoprotein Tah18-dependent NO synthesis confers high-temperature stress tolerance on yeast cells[J]. Biochemical and Biophysical Research Communications, 2013, 430(1): 137-143. DOI:10.1016/j.bbrc.2012.11.023 |
| [23] |
PARROU J L, TESTE M A, FRANÇOIS J. Effects of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: Genetic evidence for a stress-induced recycling of glycogen and trehalose[J]. Microbiology, 1997, 143(6): 1891-1900. DOI:10.1099/00221287-143-6-1891 |
| [24] |
JULES M, BELTRAN G, FRANÇOIS J, et al. New insights into trehalose metabolism by Saccharomyces cerevisiae: NTH2 encodes a functional cytosolic trehalase, and deletion of TPS1 reveals Ath1p-dependent trehalose mobilization[J]. Applied and Environmental Microbiology, 2008, 74(3): 605-614. DOI:10.1128/AEM.00557-07 |
| [25] |
ELEUTHERIO E, PANEK A, DE MESQUITA J F, et al. Revisiting yeast trehalose metabolism[J]. Current Genetics, 2015, 61(3): 263-274. DOI:10.1007/s00294-014-0450-1 |
| [26] |
KELLER F, SCHELLENBERG M, WIEMKEN A. Localization of trehalase in vacuoles and of trehalose in the cytosol of yeast (Saccharomyces cerevisiae)[J]. Archives of Microbiology, 1982, 131(4): 298-301. DOI:10.1007/BF00411175 |
| [27] |
LONDESBOROUGH J, VARIMO K. Characterization of two trehalases in baker's yeast[J]. Biochemical Journal, 1984, 219(2): 511-8. DOI:10.1042/bj2190511 |
| [28] |
DESTRUELLE M, HOLZER H, KLIONSKY D J. Isolation and characterization of a novel yeast gene, ATH1, that is required for vacuolar acid trehalase activity[J]. Yeast, 1995, 11(11): 1015-1025. DOI:10.1002/yea.320111103 |
| [29] |
AVONCE N, MENDOZA V A, MORETT E, et al. Insights on the evolution of trehalose biosynthesis[J]. BMC Evolutionary Biology, 2006, 6: 109. DOI:10.1186/1471-2148-6-109 |
| [30] |
GARRE E, MATALLANA E. The three trehalases Nth1p, Nth2p and Ath1p participate in the mobilization of intracellular trehalose required for recovery from saline stress in Saccharomyces cerevisiae[J]. Microbiology, 2009, 155(9): 3092-3099. DOI:10.1099/mic.0.024992-0 |
| [31] |
ANSELL R, GRANATH K, HOHMANN S, et al. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation[J]. EMBO Journal, 1997, 16(9): 2179-2187. DOI:10.1093/emboj/16.9.2179 |
| [32] |
HUBMANN G, GUILLOUET S, NEVOIGT E. Gpd1 and Gpd2 fine-tuning for sustainable reduction of glycerol formation in Saccharomyces cerevisiae[J]. Applied and Environmental Microbiology, 2011, 77(17): 5857-5867. DOI:10.1128/AEM.05338-11 |
| [33] |
SIDERIUS M, WUYTSWINKEL O V, REIJENGA K A, et al. The control of intracellular glycerol in Saccharomyces cerevisiae influences osmotic stress response and resistance to increased temperature[J]. Molecular Microbiology, 2000, 36(6): 1381-1390. |
| [34] |
WINKLER A, ARKIND C, MATTISON C P, et al. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress[J]. Eukaryotic Cell, 2002, 1(2): 163-173. DOI:10.1128/EC.1.2.163-173.2002 |
| [35] |
PARMAR J H, BHARTIYA S, VENKATESH K V. Characterization of the adaptive response and growth upon hyperosmotic shock in Saccharomyces cerevisiae[J]. Molecular Biosystems, 2011, 7(4): 1138-1148. DOI:10.1039/c0mb00224k |
| [36] |
PAN D Q, WIEDEMANN N, KAMMERER B. Heat stress-induced metabolic remodeling in Saccharomyces cerevisiae[J]. Metabolites, 2019, 9(11): 266. DOI:10.3390/metabo9110266 |
| [37] |
ZHANG Y Q, RAO R. Beyond ergosterol: Linking pH to antifungal mechanisms[J]. Virulence, 2010, 1(6): 551-554. DOI:10.4161/viru.1.6.13802 |
| [38] |
秦广利, 郭坤亮, 白爱琴, 等. 耐高温酵母的研究进展[J]. 酿酒科技, 2008, 10: 92-95. |
| [39] |
CASPETA L, CHEN Y, GHIACI P, et al. Altered sterol composition renders yeast thermotolerant[J]. Science, 2014, 346(6205): 75-78. DOI:10.1126/science.1258137 |
| [40] |
GODINHO C P, COSTA R, SÁ-CORREIA I. The ABC transporter Pdr18 is required for yeast thermotolerance due to its role in ergosterol transport and plasma membrane properties[J]. Environmental Microbiology, 2021, 23(1): 69-80. DOI:10.1111/1462-2920.15253 |
| [41] |
BERNER N, REUTTER K R, WOLF D H. Protein quality control of the endoplasmic reticulum and ubiquitin-proteasome-triggered degradation of aberrant proteins: Yeast pioneers the path[J]. Annual Review of Biochemistry, 2018, 87: 751-782. DOI:10.1146/annurev-biochem-062917-012749 |
| [42] |
SHAHSAVARANI H, SUGIYAMA M, KANEKO Y, et al. Superior thermotolerance of Saccharomyces cerevisiae for efficient bioethanol fermentation can be achieved by overexpression of RSP5 ubiquitin ligase[J]. Biotechnology Advances, 2012, 30(6): 1289-1300. DOI:10.1016/j.biotechadv.2011.09.002 |
| [43] |
KRSMANOVIC ' T, KÖLLING R. The HECT E3 ubiquitin ligase Rsp5 is important for ubiquitin homeostasis in yeast[J]. FEBS Letters, 2004, 577(1/2): 215-219. |
| [44] |
CUNHA J T, SOARES P O, BAPTISTA S L, et al. Engineered Saccharomyces cerevisiae for lignocellulosic valorization: A review and perspectives on bioethanol production[J]. Bioengineered, 2020, 11(1): 883-903. DOI:10.1080/21655979.2020.1801178 |
| [45] |
CARDONA F, ARANDA A, DEL-OLMO M. Ubiquitin ligase Rsp5p is involved in the gene expression changes during nutrient limitation in Saccharomyces cerevisiae[J]. Yeast, 2009, 26(1): 1-15. DOI:10.1002/yea.1645 |
| [46] |
LI P S, FU X F, CHEN M, et al. Proteomic profiling and integrated analysis with transcriptomic data bring new insights in the stress responses of Kluyveromyces marxianus after an arrest during high-temperature ethanol fermentation[J]. Biotechnology Biofuels, 2019, 12: 49. DOI:10.1186/s13068-019-1390-2 |
| [47] |
CHAMNIPA N, THANONKEO S, KLANRIT P, et al. The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8-1 for high-temperature ethanol production[J]. Brazilian Journal of Microbiology, 2018, 49(2): 378-391. DOI:10.1016/j.bjm.2017.09.002 |
| [48] |
WU W S, LI W H. Identifying gene regulatory modules of heat shock response in yeast[J]. BMC Genomics, 2008, 23(9): 439. DOI:10.1186/1471-2164-9-439 |
| [49] |
LI P S, FU X F, ZHANG L, et al. The transcription factors Hsf1 and Msn2 of thermotolerant Kluyveromyces marxianus promote cell growth and ethanol fermentation of Saccharomyces cerevisiae at high temperatures[J]. Biotechnology for Biofuels, 2017, 10: 289. DOI:10.1186/s13068-017-0984-9 |
| [50] |
GASCH A P, SPELLMAN P T, KAO C M, et al. Genomic expression programs in the response of yeast cells to environmental changes[J]. Molecular Biology of the Cell, 2000, 11(12): 4241-4257. DOI:10.1091/mbc.11.12.4241 |
| [51] |
ZARZOV P, BOUCHERIE H, MANN C. A yeast heat shock transcription factor (Hsf1) mutant is defective in both Hsc82/Hsp82 synthesis and spindle pole body duplication[J]. Journal of Cell Science, 1997, 110(6): 1879-1891. |
| [52] |
SUGIYAMA K, IZAWA S, INOUE Y. The Yap1p-dependent induction of glutathione synthesis in heat shock response of Saccharomyces cerevisiae[J]. Journal of Biological Chemistry, 2000, 275(20): 15535-15540. DOI:10.1074/jbc.275.20.15535 |
| [53] |
MORI K, KAWAHARA T, YOSHIDA H, et al. Signalling from endoplasmic reticulum to nucleus: Transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway[J]. Genes to Cells, 1996, 1(9): 803-817. DOI:10.1046/j.1365-2443.1996.d01-274.x |
| [54] |
JUNG U S, SOBERING A K, ROMEO M J, et al. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase[J]. Molecular Microbiology, 2002, 46(3): 781-789. DOI:10.1046/j.1365-2958.2002.03198.x |
| [55] |
MIYAHARA K, HIRATA D, MIYAKAWA T. YAP-1- and YAP-2-mediated, heat shock-induced transcriptional activation of the multidrug resistance ABC transporter genes in Saccharomyces cerevisiae[J]. Current Genetics, 1996, 29(2): 103-105. DOI:10.1007/BF02221572 |
| [56] |
HASHIKAWA N, SAKURAI H. Phosphorylation of the yeast heat shock transcription factor is implicated in gene-specific activation dependent on the architecture of the heat shock element[J]. Molecular and Cellular Biology, 2004, 24(9): 3648-3659. DOI:10.1128/MCB.24.9.3648-3659.2004 |
| [57] |
HASHIKAWA N, MIZUKAMI Y, IMAZU H, et al. Mutated yeast heat shock transcription factor activates transcription independently of hyperphosphorylation[J]. Journal of Biological Chemistry, 2006, 281(7): 3936-3942. DOI:10.1074/jbc.M510827200 |
| [58] |
EASTMOND D L, NELSON H C. Genome-wide analysis reveals new roles for the activation domains of the Saccharomyces cerevisiae heat shock transcription factor (Hsf1) during the transient heat shock response[J]. Journal of Biological Chemistry, 2006, 281(43): 32909-32921. DOI:10.1074/jbc.M602454200 |
| [59] |
MARTÍNEZ-PASTOR M T, MARCHLER G, SCHV-LLER C, et al. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE)[J]. EMBO Journal, 1996, 15(9): 2227-2235. DOI:10.1002/j.1460-2075.1996.tb00576.x |
| [60] |
GÖRNER W, DURCHSCHLAG E, MARTINEZ-PASTOR M T, et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity[J]. Genes Development, 1998, 12(4): 586-597. DOI:10.1101/gad.12.4.586 |
| [61] |
SMITH A, WARD M P, GARRETT S. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation[J]. EMBO Journal, 1998, 17(13): 3556-3564. DOI:10.1093/emboj/17.13.3556 |
| [62] |
ERKINA T L, LAVROVA M V, ERKIN A M. Alternative ways of stress regulation in cells of Saccharomyces cerevisiae: Transcriptional activators Msn2 and Msn4[J]. Tsitologiia, 2009, 51(3): 271-278. |
| [63] |
DELAUNAY A, PFLIEGER D, BARRAULT M B, et al. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation[J]. Cell, 2002, 111(4): 471-481. DOI:10.1016/S0092-8674(02)01048-6 |
| [64] |
GULSHAN K, LEE S S, MOYE-ROWLEY W S. Differential oxidant tolerance determined by the key transcription factor Yap1 is controlled by levels of the Yap1-binding protein, Ybp1[J]. Journal of Biological Chemistry, 2011, 286(39): 34071-34081. DOI:10.1074/jbc.M111.251298 |
| [65] |
RAITT D C, JOHNSON A L, ERKINE A M, et al. The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress[J]. Molecular Biology of the Cell, 2000, 11(7): 2335-2347. DOI:10.1091/mbc.11.7.2335 |
| [66] |
JAMIESON D J. Oxidative stress responses of the yeast Saccharomyces cerevisiae[J]. Yeast, 1998, 14(16): 1511-1527. DOI:10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S |
| [67] |
SERRANO R, KIELLAND-BRANDT M C, FINK G R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+)K+- and Ca2+-ATPases[J]. Nature, 1986, 319(6055): 689-693. DOI:10.1038/319689a0 |
| [68] |
LUTSENKO S, KAPLAN J H. Organization of P-type ATPases: Significance of structural diversity[J]. Biochemistry, 1995, 34(48): 15607-15613. DOI:10.1021/bi00048a001 |
| [69] |
PETROV V V. Functioning of yeast Pma1 H+-AT- Pase under changing charge: Role of Asp739 and Arg811 residues[J]. Biochemistry (Mosc), 2017, 82(1): 46-59. DOI:10.1134/S0006297917010059 |
| [70] |
HENDRICK J P, HARTL F U. Molecular chaperone functions of heat-shock proteins[J]. Annual Review of Biochemistry, 1993, 62: 349-384. DOI:10.1146/annurev.bi.62.070193.002025 |
| [71] |
MAYER M P. The unfolding story of a redox chaperone[J]. Cell, 2012, 148: 843-844. DOI:10.1016/j.cell.2012.02.029 |
| [72] |
JANG H H, LEE K O, CHI Y H, et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function[J]. Cell, 2004, 117(5): 625-635. DOI:10.1016/j.cell.2004.05.002 |
| [73] |
FRIGIERI M C, JOÃO LUIZ M V, APPONI L H, et al. Synthetic lethality between eIF5A and Ypt1 reveals a connection between translation and the secretory pathway in yeast[J]. Molecular Genetics and Genomics, 2008, 280(3): 211-221. DOI:10.1007/s00438-008-0357-y |
| [74] |
RICHARDSON C J, JONES S, LITT R J, et al. GTP hydrolysis is not important for Ypt1 GTPase function in vesicular transport[J]. Molecular and Cellular Biology, 1998, 18(2): 827-838. DOI:10.1128/MCB.18.2.827 |
| [75] |
KANG C H, PARK J H, LEE E S, et al. Exploring novel functions of the small GTPase Ypt1p under heat-shock by characterizing a temperature-sensitive mutant yeast strain, ypt1-G80D[J]. International Journal of Molecular Sciences, 2019, 20(1): 132. DOI:10.3390/ijms20010132 |
| [76] |
PAUMI C M, MENENDEZ J, ARNOLDO A, et al. Mapping protein-protein interactions for the yeast ABC transporter Ycf1p by integrated split-ubiquitin membrane yeast two-hybrid analysis[J]. Molecular Cell, 2007, 26(1): 15-25. DOI:10.1016/j.molcel.2007.03.011 |
| [77] |
LEE M E, SINGH K, SNIDER J, et al. The Rho1 GTPase acts together with a vacuolar glutathione S-conjugate transporter to protect yeast cells from oxidative stress[J]. Genetics, 2011, 188(4): 859-870. DOI:10.1534/genetics.111.130724 |
| [78] |
DAYER R, FISCHER B B, EGGEN R I, et al. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii[J]. Genetics, 2008, 179(1): 41-57. DOI:10.1534/genetics.107.086041 |
| [79] |
MORANO K A, GRANT C M, MOYE-ROWLEY W S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae[J]. Genetics, 2012, 190(4): 1157-1195. DOI:10.1534/genetics.111.128033 |
| [80] |
CULOTTA V C, YANG M, O'HALLORAN T V. Activation of superoxide dismutases: Putting the metal to the pedal[J]. Biochimica et Biophysica Acta, 2006, 1763(7): 747-758. DOI:10.1016/j.bbamcr.2006.05.003 |
| [81] |
HILTUNEN J K, MURSULA A M, ROTTENSTEINER H, et al. The biochemistry of peroxisomal β-oxidation in the yeast Saccharomyces cerevisiae[J]. FEMS Microbiology Reviews, 2003, 27(1): 35-64. DOI:10.1016/S0168-6445(03)00017-2 |
| [82] |
WANG Y, GIBNEY P A, WEST J D, et al. The yeast Hsp70 Ssa1 is a sensor for activation of the heat shock response by thiol-reactive compounds[J]. Molecular Biology of the Cell, 2012, 23(17): 3290-3298. DOI:10.1091/mbc.e12-06-0447 |
| [83] |
ZHENG X, KRAKOWIAK J, PATEL N, et al. Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation[J]. Elife, 2016, 5: e18638. DOI:10.7554/eLife.18638 |
| [84] |
KRAKOWIAK J, ZHENG X, PATEL N, et al. Hsf1 and Hsp70 constitute a two-component feedback loop that regulates the yeast heat shock response[J]. Elife, 2018, 7: e31668. DOI:10.7554/eLife.31668 |
| [85] |
LEACH M D, BUDGE S, WALKER L, et al. Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast[J]. PLoS Pathogens, 2012, 8(12): e1003069. DOI:10.1371/journal.ppat.1003069 |
| [86] |
MAYER M P, LE BRETON L. Hsp90:Breaking the symmetry[J]. Molecular Cell, 2015, 58(1): 8-20. DOI:10.1016/j.molcel.2015.02.022 |
| [87] |
RÖHL A, ROHRBERG J, BUCHNER J. The chaperone Hsp90:Changing partners for demanding clients[J]. Trends in Biochemical Sciences, 2013, 38(5): 253-262. DOI:10.1016/j.tibs.2013.02.003 |
| [88] |
SCHOPF F H, BIEBL M M, BUCHNER J. The HSP- 90 chaperone machinery[J]. Nature Reviews Molecular Cell Biology, 2017, 18(6): 345-360. DOI:10.1038/nrm.2017.20 |
| [89] |
AUESUKAREE C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation[J]. Journal of Bioscience and Bioengineering, 2017, 124(2): 133-142. DOI:10.1016/j.jbiosc.2017.03.009 |
| [90] |
GLOVER J R, LINDQUIST S. Hsp104, Hsp70, and Hsp40:A novel chaperone system that rescues previously aggregated proteins[J]. Cell, 1998, 94(1): 73-82. DOI:10.1016/S0092-8674(00)81223-4 |
| [91] |
STROMER T, FISCHER E, RICHTER K, et al. Analysis of the regulation of the molecular chaperone Hsp26 by temperature-induced dissociation: The N-terminal domail is important for oligomer assembly and the binding of unfolding proteins[J]. Journal of Biological Chemistry, 2004, 279(12): 11222-11228. DOI:10.1074/jbc.M310149200 |
| [92] |
HASLBECK M, MIESS A, STROMER T, et al. Disassembling protein aggregates in the yeast cytosol: The cooperation of Hsp26 with Ssa1 and Hsp104[J]. Journal of Biological Chemistry, 2005, 280(25): 23861-23868. DOI:10.1074/jbc.M502697200 |



