2. 广西中医药大学药学院, 广西南宁 530200
2. College of Pharmacy, Guangxi University of Chinese Medicine, Nanning, Guangxi, 530200, China
随着人们生活水平的不断提高以及生活方式的改变,肥胖已成为世界范围内第一大流行性疾病。目前,由于肥胖问题的严重性和危害性,亟需开发新的降脂减肥药物来有效防治。近年来,食物中的功能成分已成为肥胖预防和治疗研究的热点。一系列研究表明,岩藻黄素(Fucoxanthin)在防治肥胖方面具有显著功效[1],对胰岛素抵抗[2-4]、癌症[5-16]以及阿尔兹海默症[17]等神经退行性疾病也具有抑制和改善作用。2017年的一项人体临床试验发现,岩藻黄素具有良好的减肥作用且安全性良好[1],2020年被美国食品药品监督管理局(FDA)授予食品补充剂新膳食成分。随着研究的深入,将会进一步明确岩藻黄素的作用机制和靶点,促进岩藻黄素作为降脂减肥产品的研制与开发,加速临床上的应用进程,使肥胖、糖尿病的治疗进入一个全新的时代。
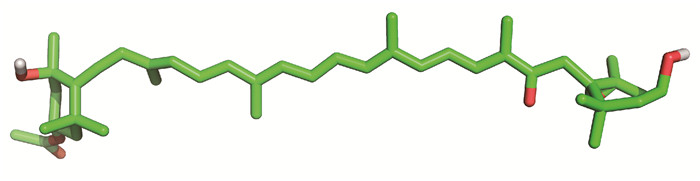
岩藻黄素,3′-乙酰氧基-6′, 7′-二脱氢-5, 6-环氧-5, 5′, 6, 6′, 7, 8-六氢-3, 5′-二羟基-8-氧代-β, β-胡萝卜素,又称褐藻素、岩藻黄质,分子式为C42H58O6,分子量为658.906,密度为1.09 g/cm3,熔点为166-168℃。岩藻黄素为粉末状固体物质,不溶于水,易溶于乙醇、石油醚、正己烷、三氯甲烷等有机溶剂,在酸性、避光和低温条件下比较稳定,而在强碱、强光或高温条件下都容易发生降解[18]。岩藻黄素的吸收峰位于450 nm,红外光谱分别在3 388,1 721 cm-1处出现羟基和羰基的特征吸收峰。与β-胡萝卜素和虾青素等其他类胡萝卜素相比,岩藻黄素的结构特征迥然不同,岩藻黄素结构中的丙二烯键和共轭双键使其具有很强的猝灭自由基活性[18]。此外,多烯烃链、单环氧基、羰基和羟基使其极易被氧化和异构化[18](图 1)。岩藻黄素分布于众多藻类[19-29](表 1)。岩藻黄素在藻类细胞中发挥光捕获和光传递作用,岩藻黄素-叶绿素a/c蛋白复合体[30]使藻类即使在弱光环境下也能够顺利进行光合作用。目前,天然岩藻黄素主要来源于海带提取物,其含量约占海带总色素量的60%以上。岩藻黄素的生物合成途径仍未被完全阐明,合成途径依次经过八氢番茄红素合成酶(PSY)、八氢番茄红素脱氢酶(PDS)、ζ-胡萝卜素脱氢酶(ZDS)、类胡萝卜素异构酶(CRTISO)和番茄红素β环化酶(LCYb)等调控,但该合成途径的最后一步仍然未知。由于岩藻黄素含量低、养殖成本高、提取工艺复杂,严重制约岩藻黄素生物合成产业的发展,造成岩藻黄素市场价格十分昂贵,生物合成产业链亟需寻求突破口。
|
| 图 1 岩藻黄素的化学结构 Fig. 1 Chemical structure of fucoxanthin |
| 编号 No. |
来源 Source |
岩藻黄素含量 Fucoxanthin content (mg/g) |
年产量 Annual yield (104 t) |
参考文献 References |
| 1 | 鼠尾藻Sargassum thunbergii | 1.37 | / | [19] |
| 2 | 海带Saccharina japonica | 0.56-2.23 | 162 | [20] |
| 3 | 羊栖菜Sargassum fusiforme | 1.07 | 2.70 | [21] |
| 4 | 牟氏角刺藻Chaetoceros muelleri | 1.31-5.02 | / | [22] |
| 5 | 柔弱角刺藻Chaetoceros debilis | 5.01 | / | [22] |
| 6 | 拟弯角刺藻Chaetoceros pseudocurvisetus | 0.03 | / | [22] |
| 7 | 骨条藻Skeletonema sp. | 0.04-0.75 | / | [22] |
| 8 | 曲壳藻Achnanthes sp. | 0.08 | / | [22] |
| 9 | 舟形藻Navicula perminuta | 0.51 | / | [22] |
| 10 | 直链藻Melosira sp. | 0.43 | / | [22] |
| 11 | 双尾藻Ditylum brightwellii | 0.41 | / | [22] |
| 12 | 冠盘藻Stephanodiscus sp. | 0.75 | / | [22] |
| 13 | 双眉藻Amphora sp. | 2.46 | / | [22] |
| 14 | 铜藻Sargassum horneri | 0.367-1.960 | / | [23] |
| 15 | 三角褐指藻Phaeodactylum tricornutum | 15.30 | / | [24-26] |
| 16 | 金色奥杜藻Odontella aurita | 9.22 | / | [27] |
| 17 | 新月菱形藻Nitzsehia closterium | 0.88 | / | [28] |
| 18 | 金藻8701 Ioschrysis 8701 | 3.23 | / | [28] |
| 19 | 湛江等鞭金藻Isochrysis zhanjiangensis | 1.80 | / | [28] |
| 20 | 海黍子Sargassum muticum | 1.16 | / | [28] |
| 21 | 裙带菜Undaria pinnatifida | 1.36 | 20.2 | [29] |
| 注:海带、羊栖菜和裙带菜年产量数据分别来自中国渔业统计年鉴(2020) (www.yearbookchina.com) Note: Annual yield data of Saccharina japonica, Sargassum fusiforme and Undaria pinnatifida from China Fisheries Statistical Yearbook(2020)(http://www.yearbookchina.com), respectively | ||||
1 岩藻黄素的消化、吸收、转运、代谢和安全性
岩藻黄素在胃肠道内被脂肪酶、胆固醇酯酶水解为岩藻黄醇,岩藻黄醇可通过淋巴系统进入血液循环运输,少量岩藻黄醇在肝脏经脱氢/异构化转化为Amarouciaxanthin A,岩藻黄醇和Amarouciaxanthin A通过异构化、脱氢、脱乙酰基、氧化和脱甲基等一系列反应,最终被分解[31-33]。也有研究认为,人和小鼠肠道吸收岩藻黄素后,由肠道细胞中的酶介导岩藻黄素酯化,酯化后的岩藻黄素被携带到包括皮肤在内的多种组织[34]。岩藻黄素作为疏水性化合物,一直被认为是通过简单扩散的方式被肠上皮细胞吸收,岩藻黄素的正辛醇-水分配系数(log P)为11.4,亲脂性高可能是其吸收有限的部分原因。影响岩藻黄素吸收速率的因素较多,包括饮食中脂质种类和摄入量,结合基质的稳定性,以及其他膳食纤维等摄入物质[35-37],将岩藻黄素与食用油或脂类结合食用,可提高岩藻黄素的吸收率[38]。由于脂溶性过高,口服岩藻黄素在人体内的生物利用度和稳定性较低[35-37]。但岩藻黄素和岩藻黄醇仍比虾青素等其他叶黄素类的利用度要高[39],用阿拉伯胶和γ-环糊精乳化岩藻黄素可以提高其稳定性和生物利用度[40]。
岩藻黄素在人体内主要被代谢为岩藻黄醇、Amarouciaxanthin A和岩藻糖黄素[31-33],其代谢物被认为是在体内发挥生理功能的主要活性形式。摄入岩藻黄素24 h,其代谢物岩藻黄醇在小鼠肝脏中经短链脱氢酶或还原酶作用进一步转化为Amarouciaxanthin A[39],然后被迅速转运至其他组织。在大鼠肝脏中,岩藻黄素的主要代谢物也为Amarouciaxanthin A[31]。岩藻黄素、岩藻黄醇、Amarouciaxanthin A在小鼠脂肪组织中的比例分别为13%、32%和55%,在其他组织(肝、肺、肾、心、脾)中占的百分比分别为1%-11%、63%-76%和20%-26%,这表明Amarouciaxanthin A主要储存在腹部白色脂肪组织,而岩藻黄醇可进入血流并储存在红细胞、肝、肺、肾、心、脾和脂肪组织中,且主要积累在小鼠肝脏和心脏[39](图 2)。
岩藻黄素对大鼠和小鼠的肝、脾、肾和性腺等均无明显损害。Kadekaru等[41]用裙带菜中岩藻黄素(纯度>95%)给模型大鼠连续灌胃28 d,未观察到大鼠有明显的毒性症状。Beppu等[42]给ICR小鼠口服岩藻黄素(纯度93%)持续30 d,单次给药剂量为1 000或2 000 mg/kg,重复给药剂量为500或1 000 mg/kg,受试鼠均未发生死亡或出现明显的外观异常;组织学观察显示,肝、肾、脾和性腺组织细胞也无异常变化。Iio等[43]研究发现,含岩藻黄素的角刺藻油提取物的半数致死量(LD50)大于2 000 mg/kg。岩藻黄素及其代谢物不具有体外致癌、致畸、致突变性[44],500-2 000 mg/kg不会引起实验小鼠畸形[45]等副作用,可用于制备浓缩食品添加剂。
2 岩藻黄素降脂作用机理岩藻黄素能耦合亚油酸,使肥胖大鼠的体重、腹内脂肪含量、甘油三脂(TG)、血糖和瘦素水平显著降低[46-48]。Hosokawa等[49]发现,岩藻黄素能够缓和Ⅱ型糖尿病动物模型KK-Ay小鼠的肥胖症,体重抑制率可达12.5%。Woo等[50]发现,岩藻黄素可显著抑制高脂饲养的C57BL/6N小鼠体重,抑制率达20%。另外,岩藻黄素还能促进脂类排泄,调控过氧化物酶体增殖物激活受体(PPARs)基因表达,干扰肝脂肪代谢酶的活性[50],降低脂肪在小鼠肝组织中的异位沉积。2017年,日本东方职业健康协会东京分会上野检测中心一项人体临床研究发现,岩藻黄素对肥胖人群具有良好的减肥作用,可改善轻微肥胖人群的体重[1]。目前的研究发现,岩藻黄素可以抑制脂肪细胞分化, 诱导白色脂肪细胞棕色化, 调节线粒体生物发生和功能运作, 调节胆固醇代谢[51, 52]。
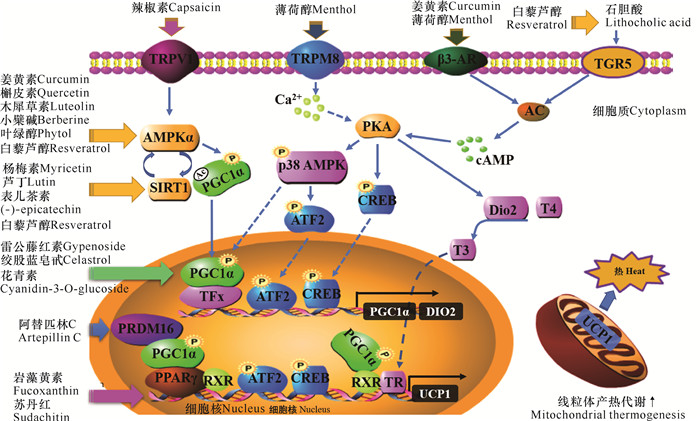
2.1 岩藻黄素抑制脂肪细胞的分化脂肪细胞分化及其调控网络与肥胖、胰岛素抵抗的关系是当前研究的热点,抑制脂肪细胞分化可以达到预防或治疗肥胖的目的。脂肪细胞分化过程涉及多转录因子的调控,起正向调控作用的转录因子主要有CCAAT/增强子结合蛋白(C/EBPs)、PPARs等;负调控转录因子主要有脂肪细胞定性和分化因子1 (ADD1),又称固醇调节元件结合蛋白1-c (SREBP1-c)[53-56](图 3)。
岩藻黄素及岩藻黄醇均可通过抑制过氧化物酶体增殖物激活受体γ (PPARγ)的表达,实现抑制脂肪细胞分化,半最大效应浓度(EC50)均小于5 μmol/L。岩藻黄素及其在白色脂肪组织中的优势代谢物均有降脂作用,其代谢物Amarouciaxanthin A也可抑制3T3-L1细胞的分化[57, 58]。岩藻黄素及其代谢物还可抑制3-磷酸甘油醛脱氢酶的活性并抑制脂滴融合,从而起到减肥效果[59-61]。
也有研究认为,岩藻黄素是PPARγ激活剂,可作为配体激活PPARγ和下游基因解耦联蛋白1 (UCP1) 等的表达,能激活白色脂肪细胞棕色化并提高产热代谢能力,如岩藻黄素可作为PPARγ完全激活剂,增强分化后3T3-L1细胞中PPARγ下游靶基因的表达[62]。岩藻黄素在3T3-L1细胞分化早期、中期和晚期具有不同的效应,即在3T3-L1细胞分化的不同时期添加岩藻黄素,其抑制脂质积累和抗肥胖效应会截然不同。Kang等[63]将3T3-L1前脂肪细胞分化分为早期(0-2 d)、中期(2-4 d)以及晚期(4 d以后) 3个阶段,在分化的早期阶段,岩藻黄素上调PPARγ、固醇调节元件结合蛋白-1(SREBP-1)和CCAAT/增强子结合蛋白α (C/EBPα)表达,促进3T3-L1细胞分化及甘油三酯的积累;在分化中、晚期阶段,岩藻黄素抑制PPARγ和C/EBPα的表达。
2.2 岩藻黄素诱导白色脂肪细胞棕色化白色脂肪细胞棕色化可促进能量消耗并限制体重增长,受PR结构域蛋白16 (PRDM16)、过氧化物酶体增殖激活受体-γ辅激活因子-1α (PGC-1α)等众多因素调控[66],这些调控因素对于探索机体能量代谢紊乱相关的肥胖、糖尿病等代谢综合征有重要意义。UCP1在褐色脂肪组织中特异性表达,是调节产热代谢的关键分子,利用UCP1使脂肪酸和葡萄糖氧化分解产生的能量以产热的形式消耗,可以促进甘油三酯的分解和利用[67, 68],抑制脂肪的过度积累,使大鼠和小鼠腹内脂肪重量显著降低[69],从而改善肥胖[4, 68, 69]。PPARγ是UCP1表达的一个重要调节元件,白色脂肪棕色化及UCP1的表达必须依赖PPARγ的完全激动剂[66, 70]。激活PPARγ可以诱导棕色脂肪特异基因的表达,促进棕色化过程[71]。岩藻黄素是PPARγ的完全激动剂之一,可增强PPARγ下游靶基因C/EBPα、PLIN、HSL、GLUT4等的表达[62]。Woo等[72]发现,膳食岩藻黄素对PPARγ的调节[60]可刺激白色脂肪组织中线粒体内膜UCP1的表达,从而诱导白色脂肪细胞棕色化[31, 65],提高有氧呼吸速率,促进线粒体再生。此外,Maeda等[4]研究表明,岩藻黄素可促进肥胖模型小鼠白色脂肪组织(WAT)中β3-肾上腺素受体Adrb3的mRNA表达,而Adrb3被认为是调节脂肪分解和产热的重要原因。
与小鼠和大鼠实验结果不同的是,0.1 μmol/L岩藻黄素及岩藻黄醇对人原代脂肪细胞没有诱导棕色化效应,岩藻黄素和岩藻黄醇对人体脂肪细胞耗氧率和UCP1、CPT-1β、GLUT4等mRNA表达均无影响,以及对PGC-1α、过氧化物酶体增殖物激活受体α (PPARα)、PPARγ、丙酮酸脱氢酶激酶4 (PDK4)、脂肪酸合成酶(FAS)及脂解酶的基因表达也无显著影响(P>0.05)[5]。
2.3 岩藻黄素调节线粒体的生物发生和功能肥胖是脂肪酸β氧化功能异常导致甘油三酯过度积累的结果,线粒体是脂肪酸β氧化的主要场所,其数量和功能调节是当前肥胖研究的新热点。岩藻黄素可猝灭氧自由基,避免生物膜的过氧化损伤,抑制肝匀浆液及线粒体中丙二醛的生成和脂质过氧化,缓解肝线粒体肿胀[2]。此外,岩藻黄素还可以增强线粒体的生物合成[73]。Jang等[74]研究发现,岩藻黄素可有效阻断花生四烯酸联合铁处理引起的细胞死亡、凋亡相关蛋白procaspase-3和多聚(ADP-核糖)聚合酶表达改变、线粒体功能障碍等病理改变。Wu等[75]研究发现,岩藻黄素能显著上调皮下腹股沟白色脂肪和附睾白色脂肪中线粒体融合蛋白MFN1、MFN2、视神经萎缩相关蛋白OPA1的表达,而对线粒体分裂蛋白FIS1的表达没有影响。Kang等[76]研究证实,岩藻黄素使高脂膳食小鼠附睾组织中腺苷酸活化蛋白激酶(AMPK)和乙酰辅酶A羧化酶(ACC)磷酸化水平下降得到改善,激活了AMPK信号通路。
2.4 岩藻黄素调节胆固醇代谢岩藻黄素在一定浓度下具有显著的降血脂功效。Jeon等[68]研究发现,海藻中的岩藻黄素不仅能够降低C57BL/6J小鼠体重和甘油三酯水平,还会使血液总胆固醇水平降低[77]。岩藻黄素(80-320 mg/kg)会使ICR雄性小鼠血浆及肝脏总胆固醇、甘油三酯及丙二醛含量显著降低,增加血清中高密度脂蛋白胆固醇(HDL-C)水平、肝脏谷胱甘肽过氧化物酶(GSH-Px)和超氧化物歧化酶(SOD)活力,其中160 mg/kg岩藻黄素效果最显著,80 mg/kg岩藻黄素效果次之[78]。但也有报道称,岩藻黄素单次给药和重复给药处理均会使ICR小鼠血浆胆固醇浓度升高。给小鼠口服岩藻黄素的剂量(500和1 000 mg/kg)远高于大鼠,但总胆固醇浓度增加的程度与大鼠实验结果相当,甚至是更高剂量的岩藻黄素,总胆固醇水平最多增长2倍。大鼠口服含0.1%的角黄素或虾青素,血清总胆固醇水平可以增加至1.8倍和1.3倍,表明部分类胡萝卜素可能会增加血清总胆固醇水平,促进胆固醇的循环再利用。也有研究发现,大鼠每天口服50 mg/kg岩藻黄素持续28 d和小鼠口服1 000 mg/kg岩藻黄素30 d,所检测的血浆、血液中的总胆固醇水平和高密度脂蛋白水平均升高[42, 43]。由以上结果可知,80-320 mg/kg低剂量岩藻黄素会使大、小鼠肝脏和血清总胆固醇含量均下降,但500和1 000 mg/kg高剂量岩藻黄素会增加大、小鼠血浆胆固醇水平,推测可能的原因是高剂量岩藻黄素作用于胆固醇代谢的某些环节,如促进胆固醇的释放和循环利用,促进高密度脂蛋白胆固醇合成等,从而导致总胆固醇水平不降反升。然而,关于岩藻黄素是否在更高剂量下增加大、小鼠体内高密度脂蛋白胆固醇水平仍需要进一步评估。
实时逆转录聚合酶链反应(RT-PCR)和蛋白免疫印迹(Western blot)验证结果表明,岩藻黄素可通过上调ICR小鼠肝脏固醇调节元件结合蛋白2(SREBP2)的表达,使其与羟甲基戊二酰辅酶A还原酶(HMG-CoA)相结合,从而抑制肝脏胆固醇的合成,同时也伴随着肝脏中HMG-CoA还原酶蛋白表达下调;通过抑制胆固醇酰基转移酶(ACAT)的表达进而抑制肝脏胆固醇的吸收;通过上调Cyp7a1基因的表达促进胆固醇转化为胆汁酸排出体外,刺激肝脏生成二十二碳六烯酸(DHA),且DHA可以帮助降低胆固醇水平[79]。此外,岩藻黄素可上调低密度脂蛋白受体(LDLR)蛋白的表达,促使低密度脂蛋白胆固醇(LDL-C)快速代谢,防止动脉粥样硬化[78, 80]。
3 其他方面的应用及作用机制岩藻黄素在其他方面应用广泛。在血糖调节方面,Maeda等[69]研究发现,岩藻黄素可减少腹内脂肪重量进而减轻肥胖,通过抑制肿瘤坏死因子表达以及降低KK-Ay小鼠血糖和血浆胰岛素的浓度,有效调节血糖水平。此外,岩藻黄素还能改善外围组织的胰岛素抗性,有效防止和改善Ⅱ型糖尿病。Maeda等[4]和Park等[81]研究发现,岩藻黄素可显著降低饮食诱导的肥胖鼠中空腹血糖浓度、血浆胰岛素水平及胰岛素抵抗指数。Woo等[50]研究发现,岩藻黄素可使高脂饲养C57BL/6N小鼠中的血糖水平、血红蛋白(A1C)和血浆胰岛素浓度显著降低,而血浆中胰高血糖素浓度无明显变化。Nishikawa等[82]发现,岩藻黄素能降低糖尿病KK-Ay小鼠的血糖水平,上调骨骼肌中葡萄糖转运蛋白-4 (GLUT4)和胰岛素受体IR的表达以及蛋白激酶B (Akt)的磷酸化水平,因此认为岩藻黄素是通过刺激胰岛素信号通路促进GLUT4表达而降低血糖含量。在成熟脂肪细胞中,岩藻黄素还可以通过抑制胰岛素受体底物1 (IRS-1)的磷酸化来抑制葡萄糖的摄取。此外,岩藻黄素在降糖、抗肿瘤、抗炎症等方面也表现出明显活性[83, 84](表 2)。
| 应用 Application |
作用机制 Mechanism of action |
| 改善阿尔兹海默症[17] Improve Alzheimer′s disease[17] |
非竞争性抑制乙酰胆碱酯酶(AChE)活性,半数抑制浓度(IC50)为81.2 μmol/L;抑制β-淀粉样蛋白的聚集及神经毒性作用 Noncompetively inhibit the AChE with IC50 of 81.2 μmol/L; inhibit aggregation and neurotoxicity of amyloid β-protein |
| 抗炎(内毒素诱导性葡萄膜炎)[6, 49, 85-87] Anti-inflammattion Endotoxin-induced uveitis[6, 49, 85-87] |
降低炎症因子如肿瘤坏死因子α (TNF-α)、前列腺素E2、白细胞介素6 (IL-6)、白细胞介素1β (IL-1β)、单核细胞趋化蛋白1 (MCP-1)、纤溶酶原激活物抑制因子1 (PAI-1)、一氧化氮(NO)的产生;抑制环氧合酶(COX-2)的过度表达和炎症反应;抑制κB抑制蛋白α (IκB-α)的细胞质降解和核转录因子κB (NF-κB)蛋白的磷酸化;抑制干扰素调节因子3 (IRF3)蛋白二聚体化,并降低IRF3磷酸化水平;抑制NF-κB通路的活化和MAPKs的磷酸化;抑制肥大细胞脱粒而产生抗炎作用 Decrease the expression of inflammatory factors, including TNF-α, prostaglandin E2, IL-6, IL-1β, MCP-1, PAI-1 and NO; inhibit the overexpression of cyclooxygenase-2 and inflammatory response; suppress IκB-α degradation or NF-κB phosphorylation; inhibit the dimerization and phosphorylation of IRF3; inactivate NF-κB and MAPKs signaling pathways; inhibite mast cell-mediated allergic inflammation |
| 抗癌及降低肿瘤耐药性(十二指肠癌、肝癌、乳腺癌、膀胱癌、胃肠腺癌、白血病、神经胶质细胞瘤)[5-16] Anti-cancer and reduce antine oplastic drug resistance (Duodenal cancer, liver cancer, breast cancer, bladder cancer, gastrointestinal adenocareinoma, leukemia, and glioma)[5-16] |
上调连接蛋白connexin 43和connexin 32的表达,增加细胞间Ca2+水平,导致细胞周期阻滞在G0/G1期,诱导凋亡;诱导活性氧ROS的积累,裂解caspases-3、caspases-7和多聚腺苷二磷酸核糖聚合酶,下调Bcl-xL表达,引发细胞凋亡;诱导GADD45基因在G1期启动细胞停滞;与阿霉素协同抑制肝癌细胞的增殖,促进凋亡 Up-regulate expression of connexin-43 and connexin-32, increase the level of intercellular Ca2+, leading to cell cycle arrest in G0/G1 phase and inducing apoptosis; induce the accumulation of reactive oxygen species (ROS), cleavage of caspases-3, caspases-7 and poly (ADP-ribose) polymerase and down-regulate Bcl-xL overexpression; induce GADD45 gene expression in G1 initiate cell arrest phase; inhibit proliferation of hepatoma cells and promote apoptosis with Adriamycin |
4 展望
多项研究结果表明,岩藻黄素及其衍生物对肥胖、胰岛素抵抗、糖尿病等代谢综合征疾病具有明显的防治效果。目前,岩藻黄素的生物活性和作用机制研究主要集中在降脂方面,如诱导白色脂肪细胞棕色化、抑制脂肪细胞分化、调节线粒体再生功能以及调节胆固醇代谢等。虽然有研究证实岩藻黄素可以影响PPARγ和UCP1的表达和活性,但其直接作用靶点还需进一步验证。另外,岩藻黄素代谢产物岩藻黄醇具有更高的生物活性,研究岩藻黄素的代谢化学对阐明岩藻黄素的降脂作用机制具有重要意义。随着岩藻黄素作用机理研究的深入和生物利用度的提高,其在医疗保健方面有广泛的应用前景。
目前,国际市场上岩藻黄素商品多为褐藻粗提物的单方和复方制剂形式,主要用作食品添加剂、保健品及化妆品,而国内尚未有合法流通的产品出现。因此,加快岩藻黄素生物合成研究(包括结构修饰与改造),降低生产成本,提高生物利用度,可为开发出活性显著、结构新颖、生物利用度更高的新型抗肥胖药物提供科学依据和保障,同时也可推动岩藻黄素的高值化开发利用。
| [1] |
HITOE S, SHIMODA H. Seaweed fucoxanthin supplementation improves obesity parameters in mild obese Japanese subjects[J]. Functional Foods in Health and Disease, 2017, 7(4): 246-262. DOI:10.31989/ffhd.v7i4.333 |
| [2] |
吴超, 任丹丹, 陈倩, 等. 海带岩藻黄素对小鼠脂质过氧化抑制作用的影响[J]. 大连海洋大学学报, 2011, 26(5): 428-431. DOI:10.3969/j.issn.1000-9957.2011.05.009 |
| [3] |
ZHANG Y P, XU W, HUANG X Q, et al. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/AKT and AMPK pathways[J]. Journal of Functional Foods, 2018, 48: 515-524. DOI:10.1016/j.jff.2018.07.048 |
| [4] |
MAEDA H, HOSOKAWA M, SASHIMA T, et al. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model[J]. Molecular Medicine Reports, 2009, 2(6): 897-902. |
| [5] |
REBELLO C J, GREENWAY F L, JOHNSON W D, et al. Fucoxanthin and its metabolite fucoxanthinol do not induce browning in human adipocytes[J]. Journal of Agricultural and Food Chemistry, 2017, 65(50): 10915-10924. DOI:10.1021/acs.jafc.7b03931 |
| [6] |
KIM K N, HEO S J, KANG S M, et al. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway[J]. Toxicology in Vitro, 2010, 24(6): 1648-1654. DOI:10.1016/j.tiv.2010.05.023 |
| [7] |
FOO S C, YUSOFF F M, IMAM M U, et al. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways[J]. Biotechnology Reports, 2019, 21: e00296. DOI:10.1016/j.btre.2018.e00296 |
| [8] |
WANG L B, ZENG Y, LIU Y, et al. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin[J]. Acta Biochimica et Biophysica Sinica, 2014, 46(10): 877-884. DOI:10.1093/abbs/gmu080 |
| [9] |
YU R X, HU X M, XU S Q, et al. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway[J]. European Journal of Pharmacology, 2011, 657(1/2/3): 10-19. |
| [10] |
徐丽华, 丁浩淼, 金旭东, 等. 岩藻黄质对人红白血病HEL细胞的凋亡作用及其机制[J]. 核农学报, 2020, 34(5): 963-972. |
| [11] |
LIU C L, HUANG Y S, HOSOKAWA M, et al. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication[J]. Chemico-Biological Interactions, 2009, 182(2/3): 165-172. |
| [12] |
KOTAKE-NARA E, ASAI A, NAGAO A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells[J]. Cancer Letters, 2005, 220(1): 75-84. DOI:10.1016/j.canlet.2004.07.048 |
| [13] |
HOSOKAWA M, KUDO M, MAEDA H, et al. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells[J]. Biochimica et Biophysica Acta, 2004, 1675(1/2/3): 113-119. |
| [14] |
YOSHIKO S, HOYOKU N. Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells[J]. In Vivo, 2007, 21(2): 305-309. |
| [15] |
朱全刚, 张树辉, 张欢欢, 等. 海藻玉壶汤及其活性成分岩藻黄质在制备治疗肝癌的药物中的应用: 201910335460.2[P]. 2019-06-14.
|
| [16] |
EID S Y, ALTHUBITI M A, ABDALLAH M E, et al. The carotenoid fucoxanthin can sensitize multidrug resistant cancer cells to doxorubicin via induction of apoptosis, inhibition of multidrug resistance proteins and metabolic enzymes[J]. Phytomedicine, 2020, 77: 153280. DOI:10.1016/j.phymed.2020.153280 |
| [17] |
何山, 崔巍, 张金荣, 等. 岩藻黄素在制备治疗阿尔茨海默病药物方面的新用途: 201510750517.7[P]. 2016-03-23.
|
| [18] |
ZHAO D, YU D, KIM M, et al. Effects of temperature, light, and pH on the stability of fucoxanthin in an oil-in-water emulsion[J]. Food Chemistry, 2019, 291: 87-93. DOI:10.1016/j.foodchem.2019.04.002 |
| [19] |
李红艳, 王璇璇, 李晓, 等. 鼠尾藻中岩藻黄素的提取工艺研究[J]. 海洋科学, 2018, 42(2): 71-77. |
| [20] |
刘梁, 勾明玥, 张春枝, 等. 海带岩藻黄素提取工艺的优化[J]. 大连工业大学学报, 2010, 29(6): 406-408. DOI:10.3969/j.issn.1674-1404.2010.06.004 |
| [21] |
尹尚军, 徐涛, 刘丽平, 等. 羊栖菜岩藻黄质的提取工艺研究[J]. 食品工业科技, 2011, 32(4): 272-275. |
| [22] |
侯红焰, 向威鹏, 张金荣, 等. 高产岩藻黄素的海洋硅藻筛选及光照条件优化[J]. 水生生物学报, 2020, 44(4): 912-919. |
| [23] |
栾青, 吕芳, 郭文, 等. 不同干藻工艺对铜藻岩藻黄素含量的影响[J]. 海洋湖沼通报, 2019(1): 102-106. |
| [24] |
KIM S M, JUNG Y J, KWON O N, et al. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum[J]. Applied Biochemistry and Biotechnology, 2012, 166(7): 1843-1855. DOI:10.1007/s12010-012-9602-2 |
| [25] |
王丽娟. 三角褐指藻高含岩藻黄质突变株的高通量筛选及评价[D]. 青岛: 青岛大学, 2018.
|
| [26] |
徐润洁, 龚一富, 陈文婷, 等. 不同发光二极管单色光质对三角褐指藻中岩藻黄素含量及相关基因表达的影响[J]. 光学学报, 2019, 39(9): 299-307. |
| [27] |
李振, 李爱芬, 张成武. 硅藻金色奥杜藻色素的HPLC分析与超临界CO2萃取研究[J]. 天然产物研究与开发, 2012, 24(6): 814-818. DOI:10.3969/j.issn.1001-6880.2012.06.023 |
| [28] |
王丽. 海藻类胡萝卜素结构鉴定及微胶囊化的研究[D]. 大连: 大连海洋大学, 2015.
|
| [29] |
苌钊, 徐小琴, 李强, 等. 超声提取裙带菜中岩藻黄素的工艺研究[J]. 药物评价研究, 2013, 36(4): 285-288. |
| [30] |
WANG W D, YU L J, XU C Z, et al. Structural basis for blue-green light harvesting and energy dissipation in diatoms[J]. Science, 2019, 363(6427): eaav0365. DOI:10.1126/science.aav0365 |
| [31] |
SANGEETHA R K, BHASKAR N, DIVAKAR S, et al. Bioavailability and metabolism of fucoxanthin in rats: Structural characterization of metabolites by LC-MS(APCI)[J]. Molecular and Cellular Biochemistry, 2010, 333(1/2): 299-310. |
| [32] |
SHIKOV A N, FLISYUK E V, OBLUCHINSKAYA E D, et al. Pharmacokinetics of marine-derived drugs[J]. Marine Drugs, 2020, 18(11): 557. DOI:10.3390/md18110557 |
| [33] |
LOURENÇO-LOPES C, FRAGA-CORRAL M, JIMENEZ-LOPEZ C, et al. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries[J]. Trends in Food Science & Technology, 2021, 117(1): 163-181. |
| [34] |
SUGAWARA T, BASKARAN V, TSUZUKI W, et al. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice[J]. The Journal of Nutrition, 2002, 132(5): 946-951. DOI:10.1093/jn/132.5.946 |
| [35] |
ASAI A, YONEKURA L, NAGAO A. Low bioavailability of dietary epoxyxanthophylls in humans[J]. British Journal of Nutrition, 2008, 100(2): 273-277. DOI:10.1017/S0007114507895468 |
| [36] |
SUN X W, XU Y, ZHAO L L, et al. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers[J]. RSC Advances, 2018, 8(61): 35139-35149. DOI:10.1039/C8RA05621H |
| [37] |
MOK I, LEE J K, KIM J H, et al. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study[J]. Food Chemistry, 2018, 258: 79-86. DOI:10.1016/j.foodchem.2018.03.047 |
| [38] |
PENG J, YUAN J P, WU C F, et al. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health[J]. Marine Drugs, 2011, 9(10): 1806-1828. DOI:10.3390/md9101806 |
| [39] |
HASHIMOTO T, OZAKI Y, TAMINATO M, et al. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice[J]. British Journal of Nutrition, 2009, 102(2): 242-248. DOI:10.1017/S0007114508199007 |
| [40] |
KUMAGAI K, NEBASHI N, MUROMACHI A, et al. Emulsified fucoxanthin increases stability and absorption in rats[J]. Nippon Shokuhin Kagaku Kogaku Kaishi, 2018, 65(7): 349-356. DOI:10.3136/nskkk.65.349 |
| [41] |
KADEKARU T, TOYAMA H, YASUMOTO T. Safety evaluation of fucoxanthin purified from Undaria pinnatifida[J]. Nippon Shokuhin Kagaku Kogaku Kaishi, 2008, 55(6): 304-308. DOI:10.3136/nskkk.55.304 |
| [42] |
BEPPU F, NIWANO Y, TSUKUI T, et al. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice[J]. Journal of Toxicological Sciences, 2009, 34(5): 501-510. DOI:10.2131/jts.34.501 |
| [43] |
IIO K, OKADA Y, ISHIKURA M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats[J]. Shokuhin Eiseigaku Zasshi, Journal of the Food Hygienic Society of Japan, 2011, 52(3): 183-189. DOI:10.3358/shokueishi.52.183 |
| [44] |
LÓPEZ-RIOS L, VEGA T, CHIRINO R, et al. Toxicological assessment of Xanthigen® nutraceutical extract combination: Mutagenicity, genotoxicity and oral toxicity[J]. Toxicology Reports, 2018, 5: 1021-1031. DOI:10.1016/j.toxrep.2018.10.007 |
| [45] |
BEPPU F, NIWANO Y, SATO E, et al. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH)[J]. The Journal of Toxicological Sciences, 2009, 34(6): 693-698. DOI:10.2131/jts.34.693 |
| [46] |
HU X J, LI Y M, LI C H, et al. Combination of fucoxanthin and conjugated linoleic acid attenuates body weight gain and improves lipid metabolism in high-fat diet-induced obese rats[J]. Archives of Biochemistry and Biophysics, 2012, 519(1): 59-65. DOI:10.1016/j.abb.2012.01.011 |
| [47] |
HOSOKAWA M. Health-promoting functions of the marine carotenoid fucoxanthin[J]. Advances in Experimental Medicine and Biology, 2021, 1261: 273-284. |
| [48] |
SETH K, KUMAR A, RASTOGI R P, et al. Bioprospecting of fucoxanthin from diatoms-challenges and perspectives[J]. Algal Research, 2021, 60: 102475. DOI:10.1016/j.algal.2021.102475 |
| [49] |
HOSOKAWA M, MIYASHITA T, NISHIKAWA S, et al. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice[J]. Archives of Biochemistry and Biophysics, 2010, 504(1): 17-25. DOI:10.1016/j.abb.2010.05.031 |
| [50] |
WOO M N, JEON S M, KIM H J, et al. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice[J]. Chemico-Biological Interactions, 2010, 186(3): 316-322. DOI:10.1016/j.cbi.2010.05.006 |
| [51] |
MERESSE S, FODIL M, FLEURY F, et al. Fucoxanthin, a marine-derived carotenoid from brown seaweeds and microalgae: A promising bioactive compound for cancer therapy[J]. International Journal of Molecular Sciences, 2020, 21(23): 9273. DOI:10.3390/ijms21239273 |
| [52] |
KOO S Y, HWANG J H, YANG S H, et al. Anti-obesity effect of standardized extract of microalga Phaeodactylum tricornutum containing fucoxanthin[J]. Marine Drugs, 2019, 17(5): 311. DOI:10.3390/md17050311 |
| [53] |
KONNO T, SASAKI K, KOBAYASHI K, et al. Indirubin promotes adipocyte differentiation and reduces lipid accumulation in 3T3-L1 cells via peroxisome proliferator-activated receptor gamma activation[J]. Molecular Medicine Reports, 2020, 21(3): 1552-1560. |
| [54] |
DE SÁ P M, RICHARD A J, HANG H, et al. Transcriptional regulation of adipogenesis[J]. Comprehensive Physiology, 2017, 7(2): 635-674. |
| [55] |
LEE J E, SCHMIDT H, LAI B B, et al. Transcriptional and epigenomic regulation of adipogenesis[J]. Molecular and Cellular Biology, 2019, 39(11): e00601-18. DOI:10.1128/MCB.00601-18 |
| [56] |
FENG S, REUSS L, WANG Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity[J]. Molecules, 2016, 21(10): 1278. DOI:10.3390/molecules21101278 |
| [57] |
SHARMA P P, BASKARAN V. Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice[J]. Algal Research (Amsterdam), 2021, 54: 102187. DOI:10.1016/j.algal.2021.102187 |
| [58] |
ROSA G P, TAVARES W R, SOUSA P M C, et al. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vivo studies and clinical trials[J]. Marine Drugs, 2019, 18(1): 8. DOI:10.3390/md18010008 |
| [59] |
YIM M J, HOSOKAWA M, MIZUSHINA Y, et al. Suppressive effects of Amarouciaxanthin A on 3T3-L1 adipocyte differentiation through down-regulation of PPAR and C/EBP alpha mRNA expression[J]. Journal of Agricultural and Food Chemistry, 2011, 59(5): 1646-1652. DOI:10.1021/jf103290f |
| [60] |
MAEDA H, HOSOKAWA M, SASHIMA T, et al. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells[J]. International Journal of Molecular Medicine, 2006, 18(1): 147-152. |
| [61] |
MAEDA H, HOSOKAWA M, SASHIMA T, et al. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues[J]. Biochemical and Biophysical Research Communications, 2005, 332(2): 392-397. DOI:10.1016/j.bbrc.2005.05.002 |
| [62] |
TAKAHASHI N, GOTO T, TAIMATSU A, et al. Bixin regulates mRNA expression involved in adipogenesis and enhances insulin sensitivity in 3T3-L1 adipocytes through PPARgamma activation[J]. Biochemical and Biophysical Research Communications, 2009, 390(4): 1372-1376. DOI:10.1016/j.bbrc.2009.10.162 |
| [63] |
KANG S I, KO H C, SHIN H S, et al. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes[J]. Biochemical and Biophysical Research Communications, 2011, 409(4): 769-774. DOI:10.1016/j.bbrc.2011.05.086 |
| [64] |
YONESHIRO T, WANG Q, TAJIMA K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44[J]. Nature, 2019, 572(7771): 614-619. DOI:10.1038/s41586-019-1503-x |
| [65] |
CHOI Y S, YU L Q. Natural bioactive compounds as potential browning agents in white adipose tissue[J]. Pharmaceutical Research, 2021, 38(4): 549-567. DOI:10.1007/s11095-021-03027-7 |
| [66] |
LORENTE-CEBRIAN S, HERRERA K, MILAGRO F I, et al. MiRNAs and novel food compounds related to the browning process[J]. International Journal of Molecular Sciences, 2019, 20(23): 5998. DOI:10.3390/ijms20235998 |
| [67] |
CHONDRONIKOLA M, VOLPI E, BORSHEIM E, et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans[J]. Cell Metabolism, 2016, 23(6): 1200-1206. DOI:10.1016/j.cmet.2016.04.029 |
| [68] |
JEON S M, KIM H J, WOO M N, et al. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice[J]. Biotechnology Journal, 2010, 5(9): 961-969. DOI:10.1002/biot.201000215 |
| [69] |
MAEDA H, HOSOKAWA M, SASHIMA T, et al. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice[J]. Journal of Agricultural and Food Chemistry, 2007, 55(19): 7701-7706. DOI:10.1021/jf071569n |
| [70] |
QIANG L, WANG L H, KON N, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ[J]. Cell, 2012, 150(3): 620-632. DOI:10.1016/j.cell.2012.06.027 |
| [71] |
AHMADIAN M, SUH J M, HAH N, et al. PPARγ signaling and metabolism: The good, the bad and the future[J]. Nature Medicine, 2013, 19(5): 557-566. DOI:10.1038/nm.3159 |
| [72] |
WOO M N, JEON S M, SHIN Y C, et al. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice[J]. Molecular Nutrition & Food Research, 2009, 53(12): 1603-1611. |
| [73] |
AHN J, HA T Y, AHN J, et al. Undaria pinnatifida extract feeding increases exercise endurance and skeletal muscle mass by promoting oxidative muscle remodeling in mice[J]. FASEB Journal, 2020, 34(6): 8068-8081. DOI:10.1096/fj.201902399RR |
| [74] |
JANG E J, KIM S C, LEE J H, et al. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress[J]. BMC Complementary and Alternative Medicine, 2018, 18(1): 97. DOI:10.1186/s12906-018-2164-2 |
| [75] |
WU M T, CHOU H N, HUANG C J. Dietary fucoxanthin increases metabolic rate and upregulated mRNA expressions of the PGC-1alpha network, mitochondrial biogenesis and fusion genes in white adipose tissues of mice[J]. Marine Drugs, 2014, 12(2): 964-982. DOI:10.3390/md12020964 |
| [76] |
KANG S I, SHIN H S, KIM H M, et al. Petalonia binghamiae extract and its constituent fucoxanthin ameliorate high-fat diet-induced obesity by activating AMP-activated protein kinase[J]. Journal of Agricultural and Food Chemistry, 2012, 60(13): 3389-3395. DOI:10.1021/jf2047652 |
| [77] |
GILLE A, STOJNIC B, DERWENSKUS F, et al. A lipophilic fucoxanthin-rich Phaeodactylum tricornutum extract ameliorates effects of diet-induced obesity in C57BL/6J mice[J]. Nutrients, 2019, 11(4): 796. DOI:10.3390/nu11040796 |
| [78] |
王添娇. 褐藻岩藻黄素对胆固醇代谢的调控作用研究[D]. 大连: 大连海洋大学, 2015.
|
| [79] |
TSUKUI T, KONNO K, HOSOKAWA M, et al. Fucoxanthin and fucoxanthinol enhance the amount of docosahexaenoic acid in the liver of KK-Ay obese/diabetic mice[J]. Journal of Agricultural and Food Chemistry, 2007, 55(13): 5025-5029. DOI:10.1021/jf070110q |
| [80] |
BEPPU F, HOSOKAWA M, NIWANO Y, et al. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-Ay mice[J]. Lipids in Health and Disease, 2012, 11(1): 112. DOI:10.1186/1476-511X-11-112 |
| [81] |
PARK H J, LEE M K, PARK Y B, et al. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice[J]. Food and Chemical Toxicology, 2011, 49(4): 727-733. DOI:10.1016/j.fct.2010.11.032 |
| [82] |
NISHIKAWA S, HOSOKAWA M, MIYASHITA K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-Ay mice[J]. Phytomedicine, 2012, 19(5): 389-394. DOI:10.1016/j.phymed.2011.11.001 |
| [83] |
BAE M, KIM M B, PARK Y K, et al. Health benefits of fucoxanthin in the prevention of chronic diseases[J]. Biochimica et Biophysica Acta Molecular and Cell Biology of Lipids, 2020, 1865(11): 158618. DOI:10.1016/j.bbalip.2020.158618 |
| [84] |
ZAHARUDIN N, STAERK D, DRAGSTED L O. Inhibition of α-glucosidase activity by selected edible seaweeds and fucoxanthin[J]. Food Chemistry, 2019, 270: 481-486. DOI:10.1016/j.foodchem.2018.07.142 |
| [85] |
刘逸煊, 刘家伶, 郭凯, 等. 岩藻黄素通过NF-κB信号通路抑制LPS诱导的细胞炎症反应的研究[J]. 福建师范大学学报: 自然科学版, 2019, 35(2): 89-96. |
| [86] |
孙培培. 微藻中抗糖基化活性成分的筛选、纯化以及抗神经炎症的研究[D]. 广州: 华南理工大学, 2018.
|
| [87] |
郭凯. 岩藻黄素抗炎及调控先天免疫机制的研究[D]. 福州: 福建师范大学, 2019.
|