2. 广西壮族自治区药用植物园, 广西南宁 530023
2. Guangxi Botanical Garden of Medicinal Plants, Nanning, Guangxi, 530023, China
相比于动物而言,植物的独特性在于其整个生命周期中具有不断产生新器官的能力。而植物分枝的形成则是植物发育的一个标志性生长特征[1],分枝的形状、位置和数量影响着植物株型的多样化及其生产力[2]。因此,植物分枝发育分化规律的研究,不仅是植物生长发育的基础问题,而且对植物的实践生产也具有重要指导意义。
植物分枝是由腋生分生组织(Axillary Meristems,AMs)分化形成的,腋生分生组织位于叶腋(叶和茎之间的连接处),发育于胚后阶段。与顶端分生组织(Shoot Apical Meristems,SAMs)相似,AMs含有多能细胞群,植物分枝取决于多能细胞的活性[3]。但这些多能细胞的维持和发育具有十分复杂的调控机制。
虽然植物激素是一类化学结构较为简单的痕量生长调节分子,却对植物的生长发育[4]和环境响应[5]都具有十分复杂的生理效应。近年来,基于新的检测工具、高通量测序技术及生物信息学开发等研究手段的应用,大量植物激素合成、信号途径等突变体被分离鉴定出来,相关的基因被克隆,使得人们在植物激素调控植物组织发育方面有了深入的了解[6],AMs发育及其影响因素等研究也得以迅猛发展。因此,本文对生长素、细胞分裂素、赤霉素和独角金内酯等植物激素在植物AMs形成与发育过程中的调控机制进行阐述,并对未来研究方向进行展望。
1 AMs的来源在AMs开始分化时,叶腋处先形成肿块,随之发育成腋芽,使分枝成为可能。AMs来源于叶腋多能细胞群的细胞分裂分化,这些细胞的体积小于相邻细胞。但AMs起源一直是个有争议的问题,目前基于细胞形态学研究,提出两种不同的AMs起源模型[7]。一种是“从头诱导”模型[8],该模型认为AMs可以在发育后期的部分或完全分化的细胞中被诱导出来。该模型是基于不定芽是从茎、叶和根中的各种成熟组织以及伤口愈伤组织中产生的事实,并有叶原基正面(靠近分生组织或上部)的表面有能力形成AMs的证据支持[8, 9]。另外一种是“分离分生组织”模型,该模型认为一些多能未分化细胞从初级顶端分生组织中分离出来并保存在叶腋中,AMs由这些细胞发育而来[8]。组织学分析发现叶原基轴上的细胞未分化[10],应用活细胞成像技术也证实拟南芥和番茄叶腋细胞中AMs来源于干细胞群[11, 12],这都为分离分生组织理论提供了支撑。但AMs祖细胞在SAMs早期处于休眠状态,只有7-9个细胞分裂,分裂率远低于非边界细胞,当AMs启动时祖细胞则会立即快速分裂[11]。
如上所述,AMs源于叶腋中的多能细胞群,这些细胞的后代形成腋芽,之后腋芽生长发育成分枝或进入休眠。可见,AMs发育过程可分为腋生分生组织的形成和腋芽的生长[13]。众所周知,植物激素在植物发育过程中起着至关重要的作用[14],如生长素、细胞分裂素、赤霉素和独角金内酯等植物激素决定了AMs形成和发育过程[15]。
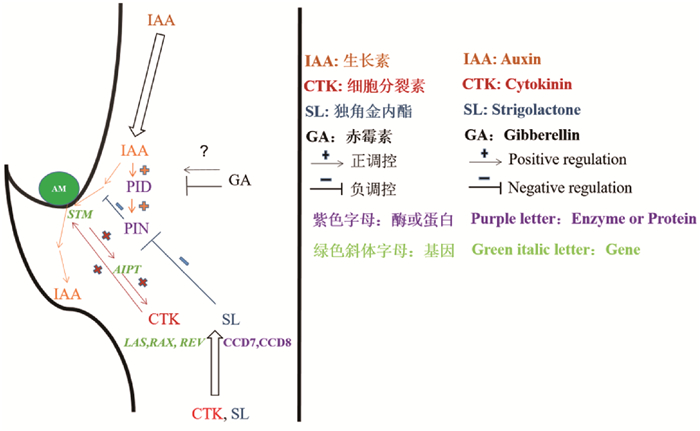
2 调控AMs形成与发育的植物激素 2.1 生长素生长素合成后经过极性运输在植物特定细胞和组织积累,调控植物生长发育[16]。当顶芽存在时,生长素自上而下运输在叶腋积累而抑制腋芽的生长,这种现象称为顶端优势[17]。可见,高浓度生长素抑制AMs的形成与发育。与此一致的是,异位表达叶腋中生长素生物合成酶iaaM抑制了拟南芥AMs的产生,而应用生长素信号抑制剂则促生腋芽[18]。DII-Venus auxin sensor的实验[19]则更直接地发现,拟南芥和番茄AMs形成的叶腋区域具有生长素浓度低的特点[20]。应用活细胞成像技术发现拟南芥叶腋细胞的SHOOT MERISTEMLESS (STM)基因的持续表达是AMs维持、激活和启动的关键,叶腋中产生过量的生长素则会降低STM的表达[12]。由此可见,叶腋中的低生长素水平是保证STM持续表达并促使AMs形成的先决条件。
顶端优势可通过打顶方式去除,在这过程中叶腋中的生长素发生了转运,而生长素转运是由生长素输出载体蛋白(PIN-FORMED,PIN)家族决定的[21]。如Wang等[20]分析拟南芥的pin1突变体的分枝表型发现,与Columbia-0 (Col-0)野生型植物相比,pin1突变体形成针状花序,产生的叶子比野生型少得多,几乎没有叶腋形成芽。生长素极性运输在pin1突变体中受阻导致高生长素水平进而抑制AMs发育,可见PIN蛋白在生长素定向运输中发挥着重要的作用[22]。而生长素响应因子(AUXIN RESPONSE FACTOR 5/MONOPTEROS,ARF5/MP)可调节PIN蛋白极性定位,从而促进生长素的不对称分布[23]。此外,丝氨酸-苏氨酸蛋白激酶(PINOID,PID)的磷酸化/去磷酸化可调节PIN蛋白极性定位[24, 25]。PID基因的表达受生长素的诱导[24],当生长素浓度升高,PID酶活性改变从而影响PIN蛋白定位,进一步影响生长素极性运输[25, 26],因此拟南芥pid突变体中的AMs也是有缺陷的[26]。总之,生长素输入和输出动态是保障叶腋中低生长素水平的关键,并促使AMs的形成与发育。
2.2 细胞分裂素除与AMs形成相关的低生长素环境外,叶腋的细胞分裂素水平也与AMs的形成与发育有着十分密切的联系。细胞分裂素信号脉冲可以通过TCS细胞分裂素信号传感器实现监测[27],应用该传感器可以在拟南芥的叶腋中心AMs启动时检测到细胞分裂素脉冲信号,并发现细胞分裂素的积累[18]。细胞分裂素是腋芽生长最有效和直接的激活剂[28],如拟南芥supershoot (sps)突变株的细胞分裂素水平上升导致侧芽的大量形成[29],外源细胞分裂素的应用促进豌豆和麻疯树AMs的形成[30, 31],等等。可见,无论是内源还是外源细胞分裂素都能积极地促进AMs形成。此外,叶腋细胞分裂素信号脉冲依赖于LATERAL SUPRESSOR(LAS)、REGULATOR OF AXILLARY MERISTEMS (RAX)和REVOLUTA (REV)等基因,如拟南芥叶腋细胞分裂素的增加使其regulator of axillary meristems (rax)突变体的AMs恢复形成[18]。Tanaka等[32]研究发现去顶后豌豆腋芽中细胞分裂素生物合成的关键酶ADENYLATE ISOPENTENYLTRANSFERASE (AIPT)基因表达水平上升。这是因为STM表达的增加在诱导AMs形成时,同时激活了AIPT的表达,促进了细胞分裂素的生物合成[33, 34]。另外,细胞分裂素生物合成[35]、细胞分裂素受体和下游B类细胞分裂素反应调节因子[18]等基因突变体的AMs形成与发育均受阻,表明细胞分裂素的生物合成、受体结合和信号传导等过程都是AMs生长发育所需的。
2.3 赤霉素赤霉素是调节植物生长发育多个方面的关键植物激素之一[36]。在多种植物中可发现赤霉素水平和AMs形成之间存在负相关关系[37-39]。在豌豆中,赤霉素对侧芽生长起抑制作用[40];拟南芥赤霉素不敏感基因gai突变体表现为顶端优势的减弱和腋芽数量的增加[41];异位表达赤霉素合成酶基因GA20ox导致叶腋赤霉素增加,抑制拟南芥AMs形成[42];而过度表达赤霉素分解代谢基因GA2ox则导致水稻[43]、番茄[44]、草坪草[45]和杨树[38]的分蘖或分枝数量增加。然而,赤霉素却能刺激柑橘和金鱼草[46]、甜樱桃[47]、麻疯树[48]以及大葱[49]的腋芽发育。目前尚不清楚这些物种是否使用相同的赤霉素信号网络来指定AMs形成的位置,或者赤霉素是否已经通过与其他激素互作而开发了替代途径,但从侧面反应赤霉素可能通过复杂的调节网络来调节AMs的形成。
2.4 独角金内酯一些突变体,如豌豆的ramosus (rms)突变体[50]、矮牵牛的decreased apical dominance (dad)突变体[51]、拟南芥的more axillary growth (max)突变体[52]以及水稻的dwarf (d)突变体[53]等,它们的生长素水平较高,木质部的细胞分裂素水平较低,但它们侧芽数目却异常增加。这与前述生长素和细胞分裂素在调节腋芽生长发育中的作用不一致[54]。通过嫁接实验可以发现,拟南芥max1、max3或max4突变体的芽嫁接到野生型根上会恢复到侧芽数少的水平,表明野生型拟南芥的根能转移某种物质抑制侧芽的生长和发育,而这类物质属于独角金内酯或其衍生物[55]。一些分枝表型增加的拟南芥突变体的独角金内酯处于低水平状态[13],再应用独角金内酯的类似物GR24则可抑制这些突变体的枝条分枝[55]。因此,独角金内酯可作为一种新的激素类应用于侧芽的生长调节方面[55, 56]。
独角金内酯最初是从根系分泌物中鉴定出来的有机化合物,能够刺激特定寄生杂草种子的萌发[57],也是营养缺乏植物根部所分泌的化学信号,可促进共生丛枝菌根真菌的菌丝分枝[58]。现已发现拟南芥的MAX3、豌豆的RMS5、水稻的D17(也称为HDT1)和矮牵牛的DAD3等基因编码的是类胡萝卜素双氧裂解酶(CAROTENOID CLEAVAGE DIOXYGEN-ASE7,CCD7)[59, 60],而拟南芥的MAX4、豌豆的RMS1、水稻的D10和矮牵牛的DAD1等基因编码的是CCD8[61, 62]。CCD7和CCD8两种酶的功能是协同将β-类胡萝卜素裂解成独角金内酯,表明独角金内酯合成途径属于类胡萝卜素合成途径[63]。水稻的ccd7和ccd8突变体缺乏独角金内酯,而外源独角金内酯应用于芽或供应到根或维管束,则能够恢复ccd7和ccd8突变体的分枝表型[55, 56]。除此之外,P450细胞色素c氧化酶(由MAX1编码)作用于MAX3和MAX4基因的下游[64],参与独角金内酯生物合成的后期步骤;而MAX2编码F-box蛋白则可能参与独角金内酯信号的接受或转导[52],因此MAX2的过表达可以一定程度抑制max3 (ccd7)和max4突变体的分枝形成[65]。
研究发现,打顶或细胞分裂素处理诱导的侧芽生长可被GR24再次抑制[66, 67],拟南芥生长素应答突变体植株可在独角金内酯作用下减少分枝数量[67]。通过对拟南芥的观察发现,独角金内酯能抑制腋芽生长是因其抑制了茎中的生长素运输流[68],而其独角金内酯突变体的生长素运输水平恢复,促进了腋芽的维管发育,进而刺激芽的生长[69]。由此可见,独角金内酯对AMs的抑制作用可能与独角金内酯抑制生长素极性运输有关。通过对比独角金内酯与N-1-Naphthylphthalamic acid (NPA)对突变或断头豌豆植物微小芽的抑制作用时发现,施用独角金内酯的腋芽会迅速停止生长,而施用NPA的则在几天后才减缓腋芽的生长。二者的区别在于独角金内酯减少了PIN1蛋白在木质部薄壁细胞基膜上的定位,并以依赖MAX2蛋白的方式减少了腋芽处生长素的输出[66],而NPA并不从细胞膜上去除PIN蛋白[70, 71],这表明独角金内酯抑制AMs可能是通过抑制PIN蛋白在细胞基膜上的定位进而影响生长素的定向转运而实现的。
2.5 激素间相互作用由前述可见,生长素、细胞分裂素、赤霉素和独角金内酯等植物激素在AMs形成和发育过程中均发挥着重要作用(图 1)。不同植物激素合成代谢途径存在着广泛的交叉反应,它们可通过多种机理实现相互作用,植物激素间这种相互作用在调控AMs形成与发育方面也有所体现。例如,当赤霉素信号阻遏蛋白SLR1与介导独角金内酯信号D14蛋白相互作用时,赤霉素信号和独角金内酯信号通路之间可能会串扰,影响水稻AMs生长发育[72]。赤霉素结合DELLA蛋白以反聚体复合物为靶点,通过非转录方式影响PIN2蛋白的液泡转运和胞吐[73],反映出赤霉素可能与生长素相互作用,但这种相互作用机制如何调节拟南芥AMs的形成尚不清楚。另外,细胞分裂素能促进生长素输出蛋白PIN3、PIN4和PIN7在拟南芥芽中的积累并进一步促进芽分枝[17, 74];赤霉素与DELLA蛋白通过物理相互作用促进拟南芥中Type-B ARABIDOPSIS RESPONSE REGULATOR (B-ARR)基因的表达和转录活性[75],Type-B ARRs则直接结合WUSCHEL (WUS)的启动子并激活WUS表达,以确定腋生分生组织形成过程中的干细胞生态位[76-78],为此,赤霉素可能通过细胞分裂素信号调节AMs的形成。独角金内酯则通过减少PIN1定位抑制生长素极性运输,从而导致拟南芥AMs发育受阻[68],但目前对于生长素运输及独脚金内酯信号途径如何共同协调侧枝发育的过程仍有待研究。近年来,油菜素内酯也被认为能调节AMs发育,如研究发现拟南芥中与油菜素内酯信号和生物合成相关的突变体中与AMs形成有关基因(如CUPSHAPED COTYLEDON, CUC和LATERAL ORGAN FUSION, LOF)的表达水平也有所改变[79, 80],但油菜素内酯是否显著影响AMs形成有待进一步确认。由此可见,植物激素对AMs发育的调控不仅仅是一种植物激素所决定的。由于具有不同的生物学功能,植物激素在调控AMs发育过程中可能存在或协作、或促进、或抑制甚至拮抗等不同的相互作用关系,由此造成植物激素对AMs发育调控的复杂性。为此,植物激素相互作用在调控AMs发育中的作用还需更加深入地研究。
|
| 图 1 植物激素调控腋生分生组织发育示意图 Fig. 1 Schematic diagram of plant hormones regulating axillary meristem development |
3 调控AMs形成与发育的其他因素
各种环境信号也参与了植物激素调控AMs发育过程。如红光与远红光比率(R∶FR)影响着植物腋芽的生长,而R∶FR是由光敏色素光感受器phyto-chrome B (phyB)感知[81]。低R∶FR或phyB功能缺失都能抑制腋芽的生长,如高粱和拟南芥phyB突变体中,植物具有较少的分枝[81, 82]。通过研究发现,R∶FR、phyB可以调节高粱中Teosinte Branche-d1 (TB1)/BRANCHED1 (BRC1) 基因表达[37, 83],当TB1/BRC1表达时会抑制D14/DAD2的OsMADS57(一种MADS盒转录因子)阻遏物[84],使得独角金内酯受体水平升高,进而抑制水稻腋芽生长。另外,phyB控制拟南芥中生长素输出载体PIN1和PIN3基因的表达[85],表明phyB可能也影响着生长素信号对AMs发育的调节。隐花素(Cryptochromes,CRYs)是一种蓝光受体,翟华伟[86]研究发现隐花素功能缺失的拟南芥cry1突变体出现多分枝表型,进一步研究发现CRY1蛋白缺失导致PIF4基因转录水平升高而使分枝增多。Domagalska等[13]认为营养缺乏也会抑制芽的生长。如土壤中低氮、低磷会导致拟南芥中细胞分裂素水平降低,分枝数减少[87, 88];而在低磷或低氮条件下生长的根及其分泌物中的独角金内酯水平增加,也伴随着高粱芽生长的减少[89]。因此,各种环境信号也参与调节AMs的发育,但是详细的机制仍然知之甚少。
4 展望综上所述,生长素、细胞分裂素、赤霉素、独角金内酯等不同植物激素的水平变化、运输及信号转导在AMs发育过程均有十分重要的作用,植物激素调控AMs发育机制也逐渐清晰。但此过程涉及多种植物激素所参与的多种生理生化代谢及基因调控,而且还受外界环境因子的影响。随着新的检测工具和分析技术的发展,未来将进一步加强植物激素的生理功能、运输、信号转导以及环境信号介导的激素互作的研究,主要包括以下4个方面:(1)发现调控AMs生长发育的新型植物激素;(2)深入探索多种植物激素调控AMs生长发育的协同或拮抗作用机制;(3)阐明如光、温、水等外界环境因子在植物激素调控AMs生长发育方面的影响,并探索环境因子引起的DNA甲基化、miRNAs剪切等表观遗传调控机制;(4)定向调控AMs生长发育实现植物生产力提升的外源植物激素应用方案研究。总之,各植物激素在环境因子参与的背景下调控AMs生长发育的机制是错综复杂的,仍需开展更加深入的研究。
| [1] |
COUDERT Y, PALUBICKI W, LJUNG K, et al. Three ancient hormonal cues co-ordinate shoot branching in a moss[J]. Elife, 2015, 4: e06808. DOI:10.7554/eLife.06808 |
| [2] |
HIRANO H Y, TANAKA W. Stem cell maintenance in the shoot apical meristems and during axillary meristem development[J]. Cytologia, 2020, 85(1): 3-8. DOI:10.1508/cytologia.85.3 |
| [3] |
LONG J, BARTON M K. Initiation of axillary and floral meristems in Arabidopsis[J]. Developmental Biology, 2000, 218(2): 341-353. DOI:10.1006/dbio.1999.9572 |
| [4] |
SANTNER A, CALDERON-VILLALOBOS L I A, ESTELLE M. Plant hormones are versatile chemical regulators of plant growth[J]. Nature Chemical Biology, 2009, 5(5): 301-307. DOI:10.1038/nchembio.165 |
| [5] |
梁莹, 李林轩, 蔡锦源, 等. 金线莲种质的生理生化特性及内源激素含量差异[J]. 广西科学院学报, 2019, 35(1): 45-50. |
| [6] |
陆维超, 赵建国, 张莉, 等. 植物茎尖分生组织分化调控机制研究进展[J]. 西北植物学报, 2016, 36(5): 1055-1065. |
| [7] |
STEEVES T A, SUSSEX I M. Patterns in plant development[M]. Cambridge, UK: Cambridge University Press, 1989: 135-144.
|
| [8] |
MCCONNELL J R, BARTON M K. Leaf polarity and meristem formation in Arabidopsis[J]. Development, 1998, 125(15): 2935-2942. DOI:10.1242/dev.125.15.2935 |
| [9] |
LYNN K, FERNANDEZ A, AIDA M, et al. The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene[J]. Development, 1999, 126(3): 469-481. DOI:10.1242/dev.126.3.469 |
| [10] |
REMPHREY W R, STEEVES T A. Shoot ontogeny in Arctostaphylos uva-ursi(bearberry): Origin and early development of lateral vegetative and floral buds[J]. Canadian Journal of Botany, 1984, 62(9): 1933-1939. DOI:10.1139/b84-264 |
| [11] |
BURIAN A, DE REUILLE P B, KUHLEMEIER C. Patterns of stem cell divisions contribute to plant longevity[J]. Current Biology, 2016, 26(11): 1385-1394. DOI:10.1016/j.cub.2016.03.067 |
| [12] |
SHI B H, ZHANG C, TIAN C H, et al. Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis[J]. PLoS Genetics, 2016, 12(7): e1006168. DOI:10.1371/journal.pgen.1006168 |
| [13] |
DOMAGALSKA M A, LEYSER O. Signal integration in the control of shoot branching[J]. Nature Reviews Molecular Cell Biology, 2011, 12(4): 211-221. DOI:10.1038/nrm3088 |
| [14] |
OLIVA M, FARCOT E, VERNOUX T. Plant hormone signaling during development: Insights from computational models[J]. Current Opinion in Plant Biology, 2013, 16(1): 19-24. DOI:10.1016/j.pbi.2012.11.006 |
| [15] |
YANG M L, JIAO Y L. Regulation of axillary meristem initiation by transcription factors and plant hormones[J]. Frontiers in Plant Science, 2016, 7: 183. DOI:10.3389/fpls.2016.00183 |
| [16] |
VANNESTE S, FRIML J. Auxin: A trigger for change in plant development[J]. Cell, 2009, 136(6): 1005-1016. DOI:10.1016/j.cell.2009.03.001 |
| [17] |
MVLLER D, LEYSER O. Auxin, cytokinin and the control of shoot branching[J]. Annals of Botany, 2011, 107(7): 1203-1212. DOI:10.1093/aob/mcr069 |
| [18] |
WANG Y, WANG J, SHI B H, et al. The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis[J]. The Plant Cell, 2014, 26(5): 2055-2067. DOI:10.1105/tpc.114.123083 |
| [19] |
VERNOUX T, BRUNOUD G, FARCOT E, et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex[J]. Molecular Systems Biology, 2011, 7(1): 508. DOI:10.1038/msb.2011.39 |
| [20] |
WANG Q, KOHLEN W, ROSSMANN S, et al. Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato[J]. The Plant Cell, 2014, 26(5): 2068-2079. DOI:10.1105/tpc.114.123059 |
| [21] |
BALLA J, KALOUSEK P, REINÖHL V, et al. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth[J]. The Plant Journal, 2011, 65(4): 571-577. DOI:10.1111/j.1365-313X.2010.04443.x |
| [22] |
WISNIEWSKA J, XU J, SEIFERTOVÁ D, et al. Polar PIN localization directs auxin flow in plants[J]. Science, 2006, 312(5775): 883. DOI:10.1126/science.1121356 |
| [23] |
BHATIA N, BOZORG B, LARSSON A, et al. Auxin acts through MONOPTEROS to regulate plant cell polarity and pattern phyllotaxis[J]. Current Biology, 2016, 26(23): 3202-3208. DOI:10.1016/j.cub.2016.09.044 |
| [24] |
CHRISTENSEN S K, DAGENAIS N, CHORY J, et al. Regulation of auxin response by the protein kinase PINOID[J]. Cell, 2000, 100(4): 469-478. DOI:10.1016/S0092-8674(00)80682-0 |
| [25] |
MICHNIEWICZ M, ZAGO M K, ABAS L, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux[J]. Cell, 2007, 130(6): 1044-1056. DOI:10.1016/j.cell.2007.07.033 |
| [26] |
FRIML J, YANG X, MICHNIEWICZ M, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux[J]. Science, 2004, 306(5697): 862-865. DOI:10.1126/science.1100618 |
| [27] |
MVLLER B, SHEEN J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis[J]. Nature, 2008, 453(7198): 1094-1097. DOI:10.1038/nature06943 |
| [28] |
SACHS T, THIMANN K V. The role of auxins and cytokinins in the release of buds from dominance[J]. American Journal of Botany, 1967, 54(1): 136-144. DOI:10.1002/j.1537-2197.1967.tb06901.x |
| [29] |
TANTIKANJANA T, YONG J W H, LETHAM D S, et al. Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene[J]. Genes&Development, 2001, 15(12): 1577-1588. |
| [30] |
LEYSER O. The control of shoot branching: An example of plant information processing[J]. Plant, Cell & Environment, 2009, 32(6): 694-703. |
| [31] |
NI J, ZHAO M L, CHEN M S, et al. Comparative transcriptome analysis of axillary buds in response to the shoot branching regulators gibberellin A3 and 6-benzyladenine in Jatropha curcas[J]. Scientific Reports, 2017, 7(1): 11417. DOI:10.1038/s41598-017-11588-0 |
| [32] |
TANAKA M, TAKEI K, KOJIMA M, et al. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance[J]. Plant Journal, 2006, 45(6): 1028-1036. DOI:10.1111/j.1365-313X.2006.02656.x |
| [33] |
JASINSKI S, PIAZZA P, CRAFT J, et al. KNOX ac-tion in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities[J]. Current Biology, 2005, 15(17): 1560-1565. DOI:10.1016/j.cub.2005.07.023 |
| [34] |
YANAI O, SHANI E, DOLEZAL K, et al. Arabidopsis KNOXI proteins activate cytokinin biosynthesis[J]. Current Biology, 2005, 15(17): 1566-1571. DOI:10.1016/j.cub.2005.07.060 |
| [35] |
MVLLER D, WALDIE T, MIYAWAKI K, et al. Cytokinin is required for escape but not release from auxin mediated apical dominance[J]. Plant Journal, 2015, 82(5): 874-886. DOI:10.1111/tpj.12862 |
| [36] |
YAMAGUCHI S. Gibberellin metabolism and its regulation[J]. Annual Review of Plant Biology, 2008, 59: 225-251. DOI:10.1146/annurev.arplant.59.032607.092804 |
| [37] |
AGUILAR-MARTÍNEZ J A, POZA-CARRIÓN C, CUBAS P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds[J]. The Plant Cell, 2007, 19(2): 458-472. DOI:10.1105/tpc.106.048934 |
| [38] |
MAURIAT M, SANDBERG L G, MORITZ T. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen[J]. Plant Journal, 2011, 67(5): 805-816. DOI:10.1111/j.1365-313X.2011.04635.x |
| [39] |
LIAO Z, YU H, DUAN J, et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice[J]. Nature Communications, 2019, 10(1): 2738. DOI:10.1038/s41467-019-10667-2 |
| [40] |
LUISI A, LORENZI R, SORCE C. Strigolactone may interact with gibberellin to control apical dominance in pea (Pisum sativum)[J]. Plant Growth Regulation, 2011, 65(2): 415-419. DOI:10.1007/s10725-011-9603-0 |
| [41] |
KOORNEEF M, ELGERSMA A, HANHART C J, et al. A gibberellin insensitive mutant of Arabidopsis thaliana[J]. Physiologia Plantarum, 1985, 65(1): 33-39. DOI:10.1111/j.1399-3054.1985.tb02355.x |
| [42] |
ZHANG Q Q, WANG J G, WANG L Y, et al. Gibberellin repression of axillary bud formation in Arabidopsis by modulation of DELLA-SPL9 complex activity[J]. Journal of Integrative Plant Biology, 2020, 62(4): 421-432. DOI:10.1111/jipb.12818 |
| [43] |
LO S F, YANG S Y, CHEN K T, et al. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice[J]. The Plant Cell, 2008, 20(10): 2603-2618. DOI:10.1105/tpc.108.060913 |
| [44] |
MARTÍNEZ-BELLO L, MORITZ T, LÓPEZ-DÍAZ I. Silencing C19-GA 2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants[J]. Journal of Experimental Botany, 2015, 66(19): 5897-5910. DOI:10.1093/jxb/erv300 |
| [45] |
AGHARKAR M, LOMBA P, ALTPETER F, et al. Stable expression of AtGA2ox1 in a low-input turf-grass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions[J]. Plant Biotechnology Journal, 2007, 5(6): 791-801. DOI:10.1111/j.1467-7652.2007.00284.x |
| [46] |
MARTH P C, AUDIA W V, MITCHELL J W. Effects of gibberellic acid on growth and development of plants of various genera and species[J]. Botanical Gazette, 1956, 118(2): 106-111. DOI:10.1086/335932 |
| [47] |
ELFVING D C, VISSER D B, HENRY J L. Gibberellins stimulate lateral branch development in young sweet cherry trees in the orchard[J]. International Journal of Fruit Science, 2011, 11(1): 41-54. DOI:10.1080/15538362.2011.554066 |
| [48] |
NI J, GAO C C, CHEN M S, et al. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas[J]. Plant and Cell Physiology, 2015, 56(8): 1655-1666. DOI:10.1093/pcp/pcv089 |
| [49] |
YAMAZAKI H, SHIRAIWA N, ITAI A, et al. Invol-vement of gibberellins in the regulation of tillering in welsh onion (Allium fistulosum L.)[J]. The Horticulture Journal, 2015, 84(4): 334-341. DOI:10.2503/hortj.MI-050 |
| [50] |
BEVERIDGE C A, ROSS J J, MURFET I C. Branching mutantrms-2 in Pisum sativum(grafting studies and endogenous indole-3-acetic acid levels)[J]. Plant Physiology, 1994, 104(3): 953-959. DOI:10.1104/pp.104.3.953 |
| [51] |
NAPOLI C. Highly branched phenotype of the petuniadad1-1 mutant is reversed by grafting[J]. Plant Physiology, 1996, 111(1): 27-37. DOI:10.1104/pp.111.1.27 |
| [52] |
STIRNBERG P, VAN DE SANDE K, LEYSER H M O. MAX1 andMAX2 control shoot lateral branching in Arabidopsis[J]. Development, 2002, 129(5): 1131-1141. DOI:10.1242/dev.129.5.1131 |
| [53] |
ISHIKAWA S, MAEKAWA M, ARITE T, et al. Suppression of tiller bud activity in tillering dwarf mutants of rice[J]. Plant and Cell Physiology, 2005, 46(1): 79-86. DOI:10.1093/pcp/pci022 |
| [54] |
BEVERIDGE C A, SYMONS G M, MURFET I C, et al. Therms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal (s)[J]. Plant Physiology, 1997, 115(3): 1251-1258. DOI:10.1104/pp.115.3.1251 |
| [55] |
GOMEZ-ROLDAN V, FERMAS S, BREWER P B, et al. Strigolactone inhibition of shoot branching[J]. Nature, 2008, 455(7210): 189-194. DOI:10.1038/nature07271 |
| [56] |
UMEHARA M, HANADA A, YOSHIDA S, et al. Inhibition of shoot branching by new terpenoid plant hormones[J]. Nature, 2008, 455(7210): 195-200. DOI:10.1038/nature07272 |
| [57] |
XIE X N, YONEYAMA K, YONEYAMA K. The strigolactone story[J]. Annual Review of Phytopathology, 2010, 48: 93-117. DOI:10.1146/annurev-phyto-073009-114453 |
| [58] |
AKIYAMA K, MATSUZAKI K, HAYASHI H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi[J]. Nature, 2005, 435(7043): 824-827. DOI:10.1038/nature03608 |
| [59] |
BOOKER J, AULDRIDGE M, WILLS S, et al. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule[J]. Current Biology, 2004, 14(14): 1232-1238. DOI:10.1016/j.cub.2004.06.061 |
| [60] |
ZOU J H, ZHANG S Y, ZHANG W P, et al. The riceHIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds[J]. The Plant Journal, 2006, 48(5): 687-698. DOI:10.1111/j.1365-313X.2006.02916.x |
| [61] |
SOREFAN K, BOOKER J, HAUROGNÉ K, et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea[J]. Genes & Development, 2003, 17(12): 1469-1474. |
| [62] |
ARITE T, IWATA H, OHSHIMA K, et al. DWARF-10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice[J]. The Plant Journal, 2007, 51(6): 1019-1029. DOI:10.1111/j.1365-313X.2007.03210.x |
| [63] |
MATUSOVA R, RANI K, VERSTAPPEN F W A, et al. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp.are derived from the carotenoid pathway[J]. Plant Physiology, 2005, 139(2): 920-934. DOI:10.1104/pp.105.061382 |
| [64] |
BOOKER J, SIEBERER T, WRIGHT W, et al. MAX1 encodes a cytochrome P450 family member that acts downstream ofMAX3/4 to produce a carotenoid-derived branch-inhibiting hormone[J]. Developmental Cell, 2005, 8(3): 443-449. DOI:10.1016/j.devcel.2005.01.009 |
| [65] |
STIRNBERG P, FURNER I J, OTTOLINE LEYSER H M. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching[J]. The Plant Journal, 2007, 50(1): 80-94. DOI:10.1111/j.1365-313X.2007.03032.x |
| [66] |
BREWER P B, DUN E A, FERGUSON B J, et al. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis[J]. Plant Physiology, 2009, 150(1): 482-493. DOI:10.1104/pp.108.134783 |
| [67] |
DUN E A, DE SAINT GERMAIN A, RAMEAU C, et al. Antagonistic action of strigolactone and cytokinin in bud outgrowth control[J]. Plant Physiology, 2012, 158(1): 487-498. DOI:10.1104/pp.111.186783 |
| [68] |
CRAWFORD S, SHINOHARA N, SIEBERER T, et al. Strigolactones enhance competition between shoot branches by dampening auxin transport[J]. Development, 2010, 137(17): 2905-2913. DOI:10.1242/dev.051987 |
| [69] |
AGUSTI J, HEROLD S, SCHWARZ M, et al. Strigo-lactone signaling is required for auxin-dependent stimulation of secondary growth in plants[J]. Proceedings of the National Academy of Sciences, 2011, 108(50): 20242-20247. DOI:10.1073/pnas.1111902108 |
| [70] |
KLEINE-VEHN J, DHONUKSHE P, SWARUP R, et al. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1[J]. Plant Cell, 2006, 18(11): 3171-3181. DOI:10.1105/tpc.106.042770 |
| [71] |
SAUER M, BALLA J, LUSCHNIG C, et al. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity[J]. Genes & Development, 2006, 20(20): 2902-2911. |
| [72] |
NAKAMURA H, XUE Y L, MIYAKAWA T, et al. Molecular mechanism of strigolactone perception by DWARF14[J]. Nature Communications, 2013, 4: 2613. DOI:10.1038/ncomms3613 |
| [73] |
SALANENKA Y, VERSTRAETEN I, LÖFKE C, et al. Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane[J]. Proceedings of the National Academy of Sciences, 2018, 115(14): 3716-3721. DOI:10.1073/pnas.1721760115 |
| [74] |
WALDIE T, LEYSER O. Cytokinin targets auxin tran-sport to promote shoot branching[J]. Plant Physiology, 2018, 177(2): 803-818. DOI:10.1104/pp.17.01691 |
| [75] |
MARÍN-DE LA ROSA N, PFEIFFER A, HILL K, et al. Genome wide binding site analysis reveals transcriptional coactivation of cytokinin-responsive genes by DELLA proteins[J]. PLOS Genetics, 2015, 11(7): e1005337. DOI:10.1371/journal.pgen.1005337 |
| [76] |
MENG W J, CHENG Z J, SANG Y L, et al. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL[J]. The Plant Cell, 2017, 29(6): 1357-1372. DOI:10.1105/tpc.16.00640 |
| [77] |
WANG J, TIAN C H, ZHANG C, et al. Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation[J]. The Plant Cell, 2017, 29(6): 1373-1387. DOI:10.1105/tpc.16.00579 |
| [78] |
ZHANG T Q, LIAN H, ZHOU C M, et al. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration[J]. The Plant Cell, 2017, 29(5): 1073-1087. DOI:10.1105/tpc.16.00863 |
| [79] |
BELL E M, LIN W C, HUSBANDS A Y, et al. Arabidopsis LATERAL ORGAN BOUNDARIES negatively regulates brassinosteroid accumulation to limit growth in organ boundaries[J]. Proceedings of the National Academy of Sciences, 2012, 109(51): 21146-21151. DOI:10.1073/pnas.1210789109 |
| [80] |
GENDRON J M, LIU J S, FAN M, et al. Brassinosteroids regulate organ boundary formation in the shoot apical meristem of Arabidopsis[J]. Proceedings of the National Academy of Sciences, 2012, 109(51): 21152-21157. DOI:10.1073/pnas.1210799110 |
| [81] |
KEBROM T H, BURSON B L, FINLAYSON S A. Phytochrome B repressesTeosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals[J]. Plant Physiology, 2006, 140(3): 1109-1117. DOI:10.1104/pp.105.074856 |
| [82] |
FINLAYSON S A, KRISHNAREDDY S R, KEBROM T H, et al. Phytochrome regulation of branching in Arabidopsis[J]. Plant Physiology, 2010, 152(4): 1914-1927. DOI:10.1104/pp.109.148833 |
| [83] |
KEBROM T H, BRUTNELL T P, FINLAYSON S A. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways[J]. Plant, Cell&Environment, 2010, 33(1): 48-58. |
| [84] |
GUO S Y, XU Y Y, LIU H H, et al. The interaction between OsMADS57 and OsTB1 modulates rice tillering viaDWARF14[J]. Nature Communications, 2013, 4: 1566. DOI:10.1038/ncomms2542 |
| [85] |
DEVLIN P F, YANOVSKY M J, KAY S A. A genomic analysis of the shade avoidance response in Arabidopsis[J]. Plant Physiology, 2003, 133(4): 1617-1629. DOI:10.1104/pp.103.034397 |
| [86] |
翟华伟. 拟南芥隐花素调控分枝发育的功能研究[D]. 北京: 中国农业科学院, 2016.
|
| [87] |
TAKEI K, UEDA N, AOKI K, et al. AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis[J]. Plant, Cell & Environment, 2004, 45(8): 1053-1062. |
| [88] |
LAN P, LI W, FISCHER R. Arabidopsis thaliana wild type, pho1, [STBZ]andpho2 mutant plants show different responses to exogenous cytokinins[J]. Plant Physiology and Biochemistry, 2006, 44(5-6): 343-350. DOI:10.1016/j.plaphy.2006.06.016 |
| [89] |
YONEYAMA K, XIE X N, KUSUMOTO D, et al. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites[J]. Planta, 2007, 227(1): 125-132. DOI:10.1007/s00425-007-0600-5 |



