2. 海南师范大学化学与化工学院, 海南海口 571158
2. College of Chemistry and Chemical Engineering, Hainan Normal University, Haikou, Hainan, 571158, China
珊瑚被称为海洋中的热带雨林,其表面和组织内部聚集着丰富的微生物资源[1]。这些微生物与珊瑚生活在一起,通过合成次级代谢产物(化学防御物质)共同抵御外敌入侵和病害防御,是药物先导结构的重要来源[2]。自20世纪90年代Dianne等[3]从一株柳珊瑚来源链霉菌Streptomyces sp. PG-19代谢产物中发现具有显著细胞毒活性的八元环内酯octalactin A以来,国内外学者从珊瑚来源微生物次级代谢产物中获得多个结构新颖、活性多样的先导化合物,为创新药物的研制提供了充分的分子基础[4, 5]。南沙群岛海域地处我国最南端,海洋微生物资源丰富,目前国内外对该海域微生物的天然产物化学研究开展得比较少,研究该海域微生物次级代谢产物的化学信息并挖掘其生物学功能对我国开发利用南沙群岛海洋微生物资源具有重要意义。本文对一株渚碧礁石珊瑚来源真菌SCAU139进行实验室的规模化发酵和次级代谢产物的化学分离鉴定,得到7个聚酮类化合物(1—7),并测试他们对苹果黑点致病菌Alternaria alternate和菠萝黑心致病菌Curvularia australiensis的抑制活性,初步揭示南沙海域海洋微生物代谢产物的化学多样性,为发现具有应用潜力的创新农药提供理论借鉴。
1 材料与方法 1.1 材料 1.1.1 出发菌株出发菌株SCAU139从南沙渚碧礁采集的石珊瑚样品中分离到,并保藏于华南农业大学海洋生物资源保护与利用粤港联合实验室。指示菌苹果黑点致病菌和菠萝黑心致病菌保藏在中国科学院南海海洋研究所海洋微生物中心。
1.1.2 改良的PDA液体培养基200 g马铃薯切成小块,加水煮沸20—30 min,用8层纱布过滤,加入20 g葡萄糖和30 g海盐,搅拌均匀,补水定容到1 000 mL,自然pH值。
1.1.3 主要仪器和设备柱层析硅胶(100—200目, 200—300目)和高效薄层制备板(HSG-FR254R),烟台江友硅胶开发有限公司生产;凝胶LH-20,Pharmacia公司生产;ESI-MS质谱仪(Finnigan LCQDECAXP HPLC-MASS),赛默飞公司生产;超导核磁共振仪(Bruker DRX-400型,内标为TMS),布鲁克公司生产;半制备型高效液相色谱仪(Agilent 1260 LC, Agilent Eclipse XDB-C18, ODS S-51T 1T 250×9.4 mm i.d.),安捷伦公司生产;旋转蒸发仪(EYELAN-1100V-W型),日本东京理化株式会社生产;液相用甲醇为色谱纯,MERCK公司生产;其他溶剂均为分析纯。
1.2 方法 1.2.1 菌种发酵从菌种保存的试管斜面上取一环在装有15 mL新鲜改良PDA固体培养基的平板中划线,放置在培养箱28℃培养3 d,再接种到装有200 mL新鲜PDA液体培养基的500 mL锥形瓶中,置于摇床28℃、200 r/min培养3 d制成种子液,按5%的接种量接种到装有400 mL新鲜PDA液体培养基的1 000 mL锥形瓶,28℃静置发酵28 d,共发酵50 L培养液。
1.2.2 化合物的提取分离取1.2.1节发酵液,经8层纱布过滤得到菌液和菌体,菌液用乙酸乙酯萃取浓缩,菌体用80%丙酮浸泡并超声提取浓缩,菌液和菌体粗提物合并得到浸膏30 g。浸膏用甲醇溶解,过正相柱层析(100—200目),氯仿-甲醇(体积比100:0—0:100)梯度洗脱,得到6个部位(Fr.1-Fr.6)。部位Fr.3过减压反相色谱柱得到5个馏分(Fr.3-1-Fr.3-5),馏分Fr.3-2过LH-20凝胶柱收集到5个小馏分(Fr.3-2-1—Fr.3-2-5),馏分Fr.3-2-2由HPLC制备(体积比45:55)得到化合物1 (5 mg,tR=15 min)和2 (8 mg,tR=10 min);馏分Fr.3-2-3过凝胶柱得到3个小馏分(Fr.3-2-3-1—Fr.3-2-3-3),馏分Fr.3-2-3-2由HPLC制备(体积比55:45)得到化合物3 (10 mg,tR=13 min)和4 (6 mg,tR=18 min);部位Fr.4继续过正相柱层析(200—300目)收集到6个馏分(Fr.4-1—Fr.4-6),馏分Fr.4-2过减压反相色谱柱得到5个馏分(Fr.4-2-1—Fr.4-2-5),小馏分Fr.4-2-3由HPLC制备(体积比60:40)得到化合物5 (13 mg,tR=16 min)和6 (9 mg,tR=21 min);小馏分Fr.4-2-4由HPLC制备(体积比60:40)得到化合物7 (7 mg,tR= 12 min)。
1.2.3 抗菌实验按照文献[6]的药敏纸片法,将苹果黑点致病菌和菠萝黑心致病菌接种到改良的PDA液体培养基中摇床培养3 d,得到的菌液超声打散并使用脱脂棉过滤。使用涂布棒将滤液均匀涂布到改良的PDA固体培养基上。样品初始浓度均为10 mg/mL,阳性对照多菌灵(CAS:10605-21-7,Aladdin)杀菌剂的初始浓度为2 mg/mL。用打孔器将滤纸制成直径6 mm的无菌小圆纸片,用定量移液枪吸取5 μL待测化合物的甲醇溶液滴加在滤纸片上,待甲醇挥发干后,将其贴于准备好的含菌固体培养基中,置于28℃培养24—48 h,观察是否产生抑菌圈。重复2次。
1.2.4 菌株鉴定海洋真菌菌株SCAU139分离自中国南沙渚碧礁石珊瑚,其总DNA按照文献[7]的方法提取。采用引物ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′)和ITS4 (5′-TCCTCCGCTTATTGATATGC -3′)对总DNA中的ITS序列进行PCR扩增,回收纯化PCR产物并委托上海美吉生物医药科技有限公司进行ITS测序测定。将测出来的ITS序列在GenBank上进行BLAST分析。
2 结果与分析 2.1 化学结构鉴定结果从一株渚碧礁石珊瑚来源真菌SCAU139的次级代谢产物中鉴定出7个聚酮类化合物,他们的结构见图 1。
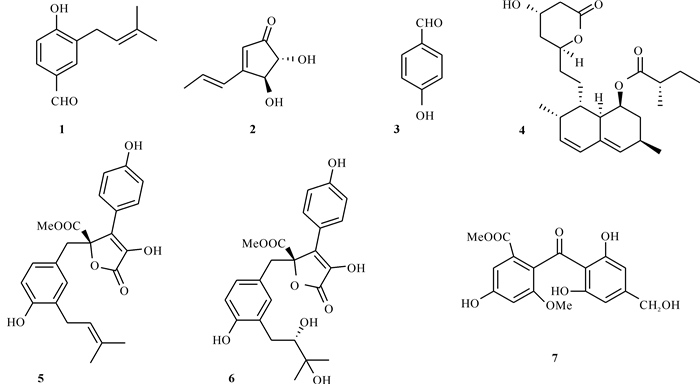
|
| 图 1 真菌SCAU139代谢产物中鉴定出的化学结构 Fig. 1 Chemical structure identified in the metabolite of SCAU139 |
2.2 单体化合物的波谱数据
化合物1:淡黄色粉末, 1H NMR (400 MHz, DMSO-d6)δH 9.73 (1H, s, H-7), 7.60 (1H, dd, J=2.0 Hz, H-2), 7.58 (1H, J=2.0, 8.0 Hz, H-6), 6.94 (1H, d, J=8.0 Hz, H-5), 5.29 (1H, m, H-9), 3.27 (2H, d, J=7.0 Hz, H-8), 1.71(3H, s, H-12), 1.67 (3H, s, H-11); 13C NMR (100 MHz, DMSO-d6) δC 190.9 (C-7), 161.8 (C-4), 132.1 (C-10), 130.6 (C-2), 130.1 (C-6), 128.5 (C-3), 127.9 (C-1), 121.9 (C-9), 115.1 (C-5), 27.7 (C-8), 25.5 (C-11), 17.6 (C-12)。经与文献[8]的数据对比, 鉴定化合物1为4-hydroxy-3-(3-methyl-2-buten-1-yl)-benzaldehyde。
化合物2:白色晶体, 1H NMR (400 MHz, DMSO-d6)δH 6.67 (1H, dq, J=7.0, 16.0 Hz, H-7), 6.33 (1H, d, J=16.0 Hz, H-6), 6.00 (1H, s, H-5), 5.77 (1H, d, J=7.0 Hz, H-3), 5.68 (1H, d, J=7.0 Hz, H-2), 4.53 (1H, brd, J=5.0 Hz, 2-OH), 3.93 (1H, brd, J=3.5 Hz, 3-OH), 1.82 (3H, d, J=7.0 Hz, 8-CH3); 13C NMR (100 MHz, DMSO-d6) δC 204.1 (C-1), 169.0 (C-4), 140.1 (C-7), 125.8 (C-6), 125.2 (C-5), 81.2 (C-2), 76.8 (C-3), 19.5 (C-8)。经与文献[9]的数据对比, 确定化合物2为土曲霉酮(terrein)。
化合物3:淡黄色粉末, 1H NMR (400 MHz, DMSO-d6) δH 9.77 (s, CHO), 7.74 (2H, d, J=8.4 Hz, H-2, H-6), 6.91 (2H, d, J=8.4 Hz, H-3, H-5);13C NMR (400 MHz, DMSO-d6) δC 190.8 (1-CHO), 163.9 (C-4), 132.1 (C-1), 128.1 (C-2, C-6), 116.0 (C-3, C-5)。经与文献[10]的数据对比, 确定化合物3为对羟基苯甲醛(4-hydroxybenzaldehyde)。
化合物4: 白色晶体, 1H NMR (400 MHz, DMSO-d6)δH 5.96 (1H, d, J=16.0 Hz, H-15), 5.80 (1H, dd, J=6.0, 9.6 Hz, H-13), 5.51 (1H, m, H-16), 5.23 (1H, m, H-10), 5.19 (1H, s, H-5), 4.49 (4.09, m, H-3), 2.65 (H-2a), 2.41 (H-2b), 1.31-1.96 (10H, m, CH2-4, CH2-6, CH2-7, CH2-11, CH2-22), 1.07 (6H, m, CH3-23, CH3-24), 0.84 (6H, m, CH3-18, CH3-19); 13C NMR (400 MHz, DMSO-d6) δC 175.5 (C-20), 170.2 (C-1), 133.1 (C-16), 131.5 (C-14), 129.1 (C-13), 128.2 (C-15), 75.8 (C-5), 67.4 (C-10), 61.3 (C-3), 40.7 (C-21), 38.5 (C-2), 36.4 (C-9), 36.0 (C-8), 35.4 (C-4), 32.4 (C-6), 31.9 (C-11), 30.1 (C-17), 26.9 (C-12), 26.3 (C-22), 23.6 (C-7), 22.6 (C-19), 16.0 (C-24), 13.6(C-18), 11.4 (C-23)。经与文献[11]的数据对比, 确定化合物4为洛伐他汀(monacolin K)。
化合物5:无色晶体, 1H NMR (400 MHz, DMSO-d6)δH 10.54 (1H, brs, 1-OH), 9.96 (1H, brs, 17-OH), 7.51 (2H, d, J=8.8 Hz, H-3, H-5), 6.89 (2H, d, J=8.8 Hz, H-2, H-6), 6.54 (1H, d, J=8.8 Hz, H-16), 6.49 (1H, dd, J=2.4, 8.4 Hz, H-15), 6.37(1H, brd, J=2.0 Hz, H-19), 5.02 (1H, m, H-21), 3.74 (3H, s, CH3-12), 3.00 (2H, m, CH2-20), 1.62 (3H, s, CH3-23), 1.53 (3H, s, CH3-24);13CNMR (100 MHz, DMSO-d6) δC 169.9 (C-11), 167.9 (C-9), 157.9 (C-1), 153.8 (C-17), 138.1 (C-8), 131.4 (C-22), 130.9 (C-19), 128.8 (C-3, C-5), 128.4 (C-15), 127.5 (C-7), 126.5 (C-18), 123.1(C-14), 122.4 (C-21), 121.1 (C-4), 115.8 (C-2, C-6), 114.1 (C-16), 84.7 (C-10), 53.5 (C-12), 38.1 (C-13), 27.5 (C-20), 25.5 (C-23), 17.5 (C-24)。经与文献[12]的数据对比, 确定化合物5为butyrolactone Ⅰ。
化合物6:淡黄色油状物, 1H NMR (400 MHz, CDCl3) 7.62 (2H, d, J=8.8 Hz, H-2′, H-6′), 6.91 (2H, d, J=8.8 Hz, H-3″, H-5″), 6.65 (1H, s, H-2″), 6.55 (1H, dd, J=2.0, 8.4 Hz, H-6″), 6.49 (1H, d, J=8.4 Hz, H-5″), 4.52 (1H, t, J=8.8 Hz, H-8″), 3.77 (3H, s, 5-OCH3), 3.57 (1H, d, H-6α), 3.49 (1H, d, H-6β), 3.04 (2H, m, CH2-7″), 1.28 (3H, s, CH3-10″), 1.15(3H, s, CH3-11″); 13CNMR (100 MHz, CDCl3)δC 169.7 (C-5), 169.2 (C-1), 158.8 (C-4″), 156.6 (C-4′), 137.3 (C-2), 130.1(C-6″), 129.5 (C-2′, C-6′), 128.0 (C-3), 126.9 (C-2″), 126.8 (C-3″), 124.7 (C-1″), 122.2 (C-1′), 116.1(C-3′, C-5′), 108.5 (C-5″), 89.2 (C-8″), 86.0 (C-4), 72.3 (C-9″), 53.6 (5-OCH3), 38.9 (C-6), 30.5 (C-7″), 25.9 (C-10″), 23.9 (C-11″)。经与文献[13]的数据对比, 确定化合物6为pernolide D。
化合物7:淡黄色粉末, 1H NMR (400 MHz, DMSO-d6)δH 6.90 (1H, d, J=2.0 Hz, H-6), 6.69 (1H, d, J=2.0 Hz, H-4), 6.23 (2H, s, H-3′ and H-5′), 4.35 (2H, s, 4′-CH2OH), 3.64 (3H, s, 3-OCH3), 3.63 (3H, s, 1-COOCH3); 13C NMR (100 MHz, DMSO-d6)δC 199.9 (C-7), 165.7 (1-COOCH3), 161.8 (C-2′, C-6′), 158.2 (C-5), 156.8 (C-3), 152.2(C-4′), 127.9 (C-1), 126.2 (C-6), 109.8 (C-1′), 107.2 (C-3′, C-5′), 104.1 (C-2), 103.5 (C-4), 62.4 (4′-CH2OH), 56.0 (3′-OCH3), 52.1 (1-COOCH3)。经与文献[14]的数据对比, 确定化合物7为hydroxysulochrin。
2.3 菌株的鉴定海洋真菌菌株SCAU139的ITS序列在GenBank上进行BLAST分析,发现该序列与已知菌株Aspergillus terreus KU743892的相似度为99%,同时结合真菌形态学分析(图 2),鉴定菌株SCAU139为A.terreus。
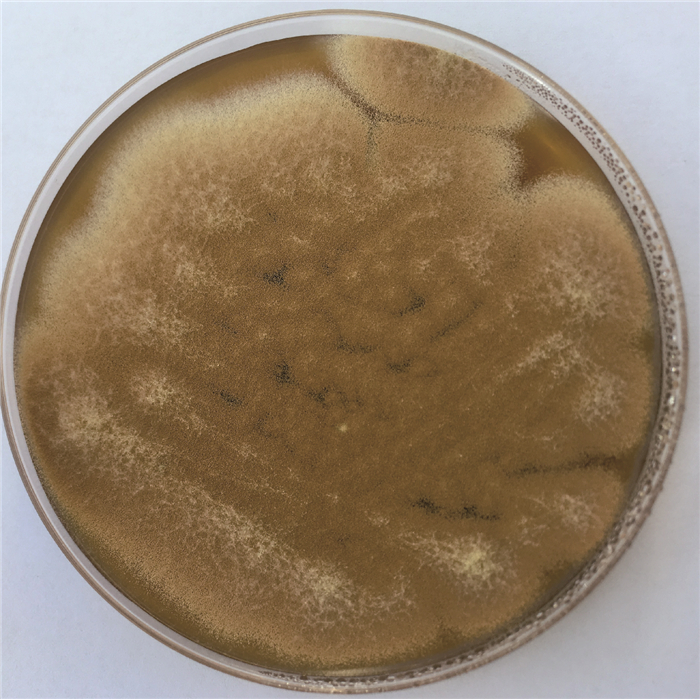
|
| 图 2 真菌A.terreus SCAU139的形态学特征 Fig. 2 Morphological characteristics of fungal strain A.terreus SCAU139 |
2.4 抗真菌实验结果
对分离到的7个单体化合物,采用滤纸片法测试其对苹果黑点致病菌和菠萝黑心致病菌的抑制活性。结果显示:化合物1—7对上述2种真菌的生长无明显的抑制活性,阳性对照多菌灵能较显著抑制这2种真菌的活性,抑制圈直径约为20 mm。
3 结论对一株南沙石珊瑚来源真菌A.terreus SCAU139进行次级代谢产物的化学研究,得到7个聚酮类化合物,化合物2在其他海洋真菌菌株的代谢产物中也有发现[15-17],暗示其具有一定的化学生态学意义。初步测试7个化合物对苹果黑点致病菌Alternaria alternate和菠萝黑心致病菌Curvularia australiensis的抑制活性,结果表明他们没有明显的抗真菌活性。
| [1] |
MEGAN J H, AMY A. Coral microbiome database:Integration of sequences reveals high diversity and relatedness of coral-associated microbes[J]. Environmental Microbiology Reports, 2019, 11(3): 372-385. DOI:10.1111/1758-2229.12686 |
| [2] |
SANG V T, TAD T T, VINH L B, et al. Coral and coral-associated microorganisms:A prolific source of potential bioactive natural products[J]. Mar Drugs, 2019, 17(8): 468. DOI:10.3390/md17080468 |
| [3] |
DIANNE M T, MARK R, WILLIAM F, et al. Octalactins A and B:Cytotoxic eight-membered-ring lactones from a marine bacterium, Streptomyces sp.[J]. Journal of the American Chemical Society, 1991, 113(12): 4682-4683. DOI:10.1021/ja00012a048 |
| [4] |
HOU X M, HAI Y, GU Y C, et al. Chemical and bioactive marine natural products of coral-derived microorganisms (2015-2017)[J]. Current Medicinal Chemistry, 2019, 26(38): 6930-6941. DOI:10.2174/0929867326666190626153819 |
| [5] |
HOU X M, XU R F, GU Y C, et al. Biological and chemical diversity of coral-derived microorganisms[J]. Current Medicinal Chemistry, 2015, 22(32): 3707-3762. DOI:10.2174/0929867322666151006093755 |
| [6] |
ACAR J F, GOLDSTEIN F W, KITZIS M D, et al. Resistance pattern of anaerobic bacteria isolated in a general hospital during a two-year period[J]. Journal of Antimicrobial Chemotherapy, 1981, 8(Suppl D): 9-16. DOI:10.1093/jac/8.suppl_D.9 |
| [7] |
LAI X T, CAO L X, TAN H M, et al. Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea[J]. The ISME Journal, 2007, 1(8): 756-762. DOI:10.1038/ismej.2007.51 |
| [8] |
VU M, HERFINDAL L, JUVIK O J, et al. Toxic aromatic compounds from fruits of Narthecium ossifragum L.[J]. Phytochemistry, 2016, 132: 76-85. DOI:10.1016/j.phytochem.2016.09.010 |
| [9] |
梁廷梅, 牟晓凤, 王聪, 等. 1株南沙群岛柳珊瑚来源真菌Aspergillus terreus中化合物及mPTPB酶抑制活性研究[J]. 中国海洋药物, 2016, 35(4): 35-39. |
| [10] |
李红霞, 邓铁忠, 陈玉, 等. 灯心草酚性成分的分离与结构鉴定[J]. 药学学报, 2007, 42(2): 174-178. |
| [11] |
ENDO A, NEGISHI Y, IWASHITA T, et al. Biosynthesis of ML-236B (Compactin) and monacolin K[J]. The Journal of Antibiotics, 1985, 38(3): 444-448. |
| [12] |
RAO K V, SADHUKHAN A K, VEERENDER M, et al. Butyrolactones from Aspergillus terreus[J]. Chemical & Pharmaceutical Bulletin, 2000, 48(4): 559-562. |
| [13] |
PAULWATT N, DAMRONG S, NATTAWUT B, et al. Butenolide and furandione from an endophytic Aspergillus terreus[J]. Chemical & Pharmaceutical Bulletin, 2010, 58(9): 1221-1223. |
| [14] |
ATSUMI S, CHISAKO S, MIYAKO K, et al. Hydroxysulochrin, a tea pollen growth inhibitor from the fungus Aureobasidium sp.[J]. Bioscience, Biotechnology, and Biochemistry, 2003, 67(2): 442-444. DOI:10.1271/bbb.67.442 |
| [15] |
PARVATKAR R R, D'SOUZA C, TRIPATHI A, et al. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus[J]. Phytochemistry, 2009, 70(1): 128-132. |
| [16] |
LI H L, LI X M, YANG S Q, et al. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531[J]. The Journal of Antibiotics, 2020, 73(2): 108-111. |
| [17] |
HAROON M H, PREMARATNE S R, CHOUDHRY M I, et al. A new β-glucuronidase-inhibiting butyrolactone from the marine endophytic fungus Aspergillus terreus[J]. Natural Product Research, 2013, 27(12): 1060-1066. |



