2. 广西大学物理科学与工程技术学院,广西高校新能源材料及相关技术重点实验室,广西南宁 530004
2. Guangxi Colleges and Universities Key Laboratory of Novel Energy Materials and Related Technology, College of Physics Science and Engineering, Guangxi University, Nanning, Guangxi, 530004, China
Iron-aluminides have attracted a lot of interests due to their high melting point, high strength, good mechanical properties, excellent oxidation, corrosion resistance and low costs[1-3].On the other hand, iron-aluminide has also been considered for applications as heating elements owing to its high electrical resistivity[4] and magnetic materials for its outstanding magnetic performance.Most studies focus on B2-FeAl alloys.
B2-FeAl exist over a wide range of compositions, from 32 at% to 50 at% Al at room temperature and from 23.5 at% to 51 at% Al at elevated temperatures[5].Thermal defects exist at all finite temperatures and the off-stoichiometric compositions generate the constitution defects, which are dominating the total defect concentration at ambient temperatures.The point defect structure in B2-FeAl is probably of the triple defect type[6-7] having Fe anti-site atom for Fe-rich composition, Fe vacancy for Al-rich composition, and a combination of two Fe vacancies and one Fe anti-site atom at stoichiometric composition.In addition to structural defects needed to accommodate deviations from stoichiometry, several percents of Fe antisite and Fe vacancy defects were found due to lattice disorder, with greater disorder in Fe-deficient alloys[8].
The technological application of iron aluminides is also impaired by their low tensile ductility at ambient temperatures.Many efforts have been devoted to the understanding of ductility properties in B2-FeAl, e.g.quenching-in vacancy hardening[9-11], environmental embrittlement[12] and its intrinsic brittleness[13-14].The improvement of brittleness for Fe-Al alloys depends on a thorough understanding of their deformation mechanisms in relation to the defect structures and concentration in these materials.Experimental as well as theoretical studies[9-11, 15-17]suggest that the microhardness is also in correlation with vacancy concentration.However, the values of vacancy concentration, especially the dependence of concentration on Al composition, and microhardness are scarcity in the literature.
What is the effect of Al composition on the point defect concentration and microhardness for B2-Fe1-xAlx? The aim of the present work is to experimentally determine the vacancy concentration in B2-FeAl alloys as functions of Al composition and annealing temperatures.The microhardness and the relationship between vacancy and microhardness have been investigated to understand the mechanism of vacancy hardening for B2-FeAl.
1 Experimental procedureFe1-xAlx(x=0.40, 0.42, 0.45, 0.47, 0.49, 0.50 and 0.51) alloys were prepared with pure Al and Fe (99.99 wt.%) by arc melting, using a non-consumable tungsten electrode and a water-cooled copper tray under an atmosphere of purified argon (99.99 wt%).During the melting process, Ti was used as an oxygen getter.The samples were remelted more than four times for good homogenization.For all of the samples, the weight loss was less than 0.5 wt% after melting, which can be considered to be negligible.All samples were sealed in evacuated quartz tubes for heat treatment.All samples were annealed at 773 K for 168 h, 873 K for 144 h, 973 K for 120 h, 1 073 K for 96 h and 1 173 K for 72 h, respectively and finally quenched into ice-water mixture.The annealed samples were powdered for x-ray diffraction (XRD) analysis, which was performed on a Dt 3500 diffractometer with a copper target, graphite monochromator, with a voltage of 36 kV and a current of 25 mA.The mass density was measured by the hydrostatic technique.The microhardness measurements were performed by Hv-1000 with 0.98 N load and held for 15 s.The effective formation energy of vacancy was evaluated with embedded atom method (EAM) [15].
2 Results and discussionFig. 1 shows the lattice constants for Fe1-xAlx alloys after annealing at temperature 773 K.The other experimental results were also included for comparison.One can see that the present results are coincident with those of Kogachi et al[11], but larger than those of Chang et al[10], Schneibel et al[17] and Hanc et al[18], but less than those of Zhao et al[19] and Gialanella et al[20].The agreement between the present results and other experimental measurement indicates that present lattice constants of Fe1-xAlx alloys are reliable.Fig. 2 demonstrates the lattice constants of Fe1-xAlx alloys after annealing at temperature of 773 K, 873 K, 973 K, 1 073 K and 1 173 K, respectively.

|
Fig.1 The lattice constants of or Fe1-xAlx alloys after annealing at temperature of 773 K |
|
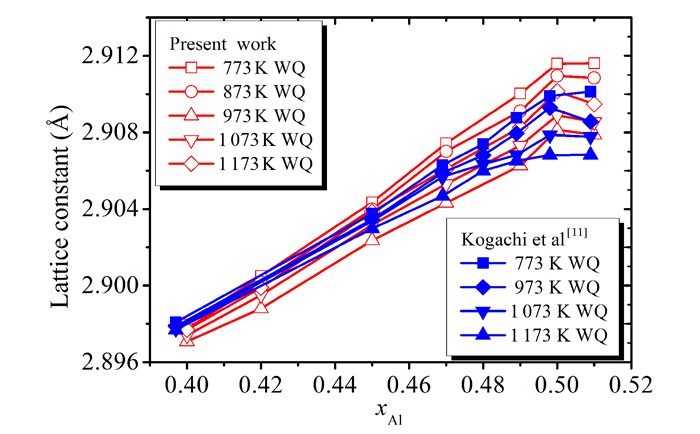
Fig.2 The lattice constants of Fe1-xAlx alloys after different annealing temperatures |
The results of Kogachi et al[11] are also included for comparison.One can see that the lattice constants increase with the Al content and annealing temperature when the x≤0.5 and then decrease.It is obvious that the present results are in reasonable agreement with those of Kogachi et al[11].
The vacancy concentrations are determined from the room temperature measurements of the XRD density and bulk density of specimens quenched from a particular temperature as
| $ {C_{\rm{V}}} = \frac{{{\rho _x}-{\rho _b}}}{{{\rho _b}}}, $ | (1) |
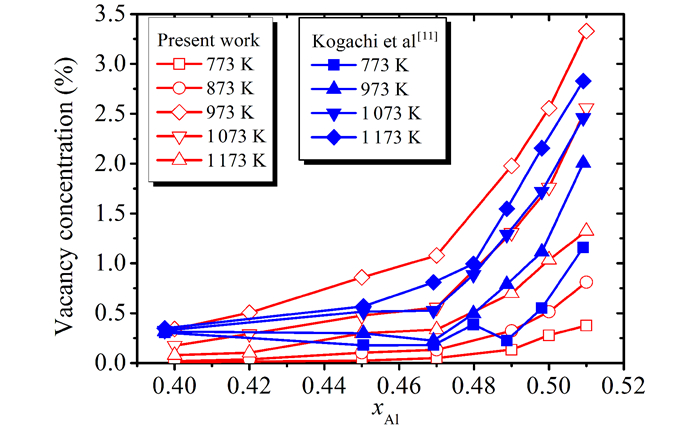
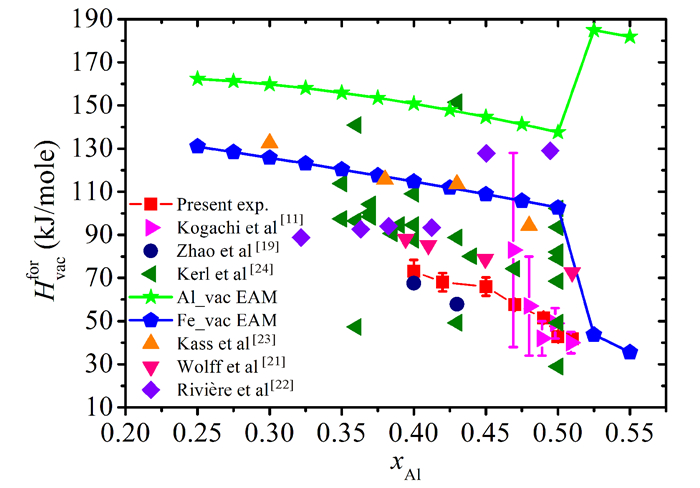
where CV is the vacancy concentration defined as NV/N(where NV and N are the total numbers of vacancies and atoms, respectively), ρx is the so called XRD density determined from the lattice constant and the nominal composition, and ρb is the bulk density measured by hydrostatic method.The vacancy concentrations of Fe1-xAlx alloys after annealing at different temperatures are plotted in Fig. 3.For comparison, the results of Kogachi et al[11] are also included in Fig. 3.It can be seen that the vacancy concentration increases with Al content and the annealing temperature.Though the vacancy concentration in this work is slightly larger than those of Kogachi et al[11] and Hanc et al[18], the changing tendency of the vacancy concentration versus Al content and annealing temperatures is in good agreement with that of Kogachi et al[11].The effective vacancy formation enthalpies of Fe1-xAlx alloys obtained by present experiments and EAM prediction are plotted in Fig. 4.One can see that the effective formation enthalpy of vacancy for Fe1-xAlx alloys is scattered.The present experimental results are close to those of Kogachi et al[11], and larger than those of Zhao et al[19] but smaller than those of Wolff et al[21], Rivière et al[22] and Kass et al[23].The results of EAM indicate that the formation enthalpy of Fe vacancy is less than that of Al vacancy and the magnitude of formation enthalpy decreases with Al content and changes suddenly and reversely for Al vacancy and Fe vacancy at stoichiometric composition.Both of experimental values[24] and the data evaluated with embedded atom method[15] indicate that the formation enthalpy changes on a large scale.Theoretical and experimental formation enthalpies decrease with the Al content on the whole, this results in the increase of vacancy concentration.
|
Fig.3 The vacancy concentration of Fe1-xAlx alloys after different annealing temperatures |
|
Fig.4 The effective formation enthalpy of vacancy for Fe1-xAlx alloys |
In order to determine the effect of vacancy concentration on the property of Fe1-xAlx alloys, the microhardness was measured and the results are displayed in Fig. 5.The microhardness increases with Al content and annealing temperature.From the figure, one can see that the present values are less than the results of Skoglund et al[25].Their results present that the microhardness for Fe68Al32 sample annealed at different temperatures are the same.The present results are also less than those of Chang et al[10] and Gialanella et al[20].In the whole, the changing tendency of microhardness as function of Al composition agrees with that of Chang et al[10].

|
Fig.5 The microhardness Fe1-xAlx alloys as a function of Al composition from material heat treated and quenched from high temperatures |
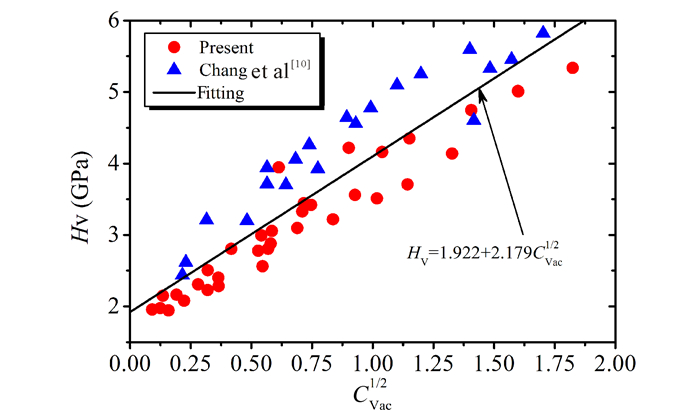
To insight the effect of vacancy concentration on microhardness or the mechanism of vacancy hardening, the microhardness was plotted as a function of square root of vacancy concentration, Cv and it is plotted in Fig. 6.It is noticeable that there is a good linear relationship between microhardness and square root of vacancy concentration, which is in good agreement with the conclusion of Chang et al[10].One should bear in mind that there exists a large amount of Fe antisite constitutional defect as the Al composition decreases, which is independent on annealing temperature, and the hardening mechanism has a dispute[26].However, the present results successfully confirm the relationship between the microhardness and vacancy concentration.This suggests the vacancies have a more significant effect on the microhardness than the Fe antisite-atoms.The relationship between the microhardness and vacancy concentration can be fitted with a formula as, HV=1.922+2.179CVac1/2.This also indicates that the hardening mechanism of B2-FeAl is vacancy hardening, namely, the interaction between the vacancies and dislocations.
|
Fig.6 Relationship between microhardness and vacancy concentration, the line is fitted to all experimental data |
The quenched-in vacancy concentration in B2-Fe1-xAlx has been obtained as functions of composition (0.4≤x≤0.51) and quenching temperature ranging from 773 K to 1 173 K by XRD and bulk density measurements.The vacancy concentration increases slowly with Al when the Al content deviated far from the stoichiometric composition and then increases rapidly as approaching the stoichiometric composition.The measured microhardness increases monotonously with vacancy concentration, which demonstrates the hardening mechanism of B2-FeAl alloys is vacancy hardening.The quantitative relationship between the microhardness and vacancy concentration can be formulated as HV=1.922+2.179CVac1/2.
| [1] | JORDAN J L, DEEVI S C. Vacancy formation and effects in FeAl[J]. Intermetallics, 2003, 11(6):507–528. DOI:10.1016/S0966-9795(03)00027-X |
| [2] | STOLOFF N S. Iron aluminides:Present status and future prospects[J]. Materials Science and Engineering:A, 1998, 258(1/2):1–14. |
| [3] | YAMAGUCHI M, INUI H, ITO K. High-temperature structural intermetallics[J]. Acta Materialia, 2000, 48(1):307–322. DOI:10.1016/S1359-6454(99)00301-8 |
| [4] | LILLY A C, DEEVI S C, GIBBS Z P. Electrical properties of iron aluminides[J]. Materials Science and Engineering:A, 1998, 258(1/2):42–49. |
| [5] | SUNDMAN B, OHNUMA I, DUPIN N, et al. An asse-ssment of the entire Al-Fe system including D03 ordering[J]. Acta Materialia, 2009, 57(10):2896–2908. DOI:10.1016/j.actamat.2009.02.046 |
| [6] | NEUMANN J P. On the occurrence of substitutional and triple defects in intermetallic phases with the B2 structure[J]. Acta Metallurgica, 1980, 28(8):1165–1170. DOI:10.1016/0001-6160(80)90099-1 |
| [7] | WASILEWSKI R J. Structural defects in CsCl intermetallic compounds—Ⅰ.Theory[J]. Journal of Physics and Chemistry of Solids, 1968, 29(1):39–49. DOI:10.1016/0022-3697(68)90252-7 |
| [8] | COLLINS G S, PENG L S J. Point defects in FeA[J]. IL Nuovo Cimento, 1996, 18D(2/3):329–336. |
| [9] | PIKE L M, CHANG Y A, LIU C T. Point defect concentration and hardening in binary B2 intermetallics[J]. Acta Materialia, 1997, 45(9):3709–3719. DOI:10.1016/S1359-6454(97)00028-1 |
| [10] | CHANG Y A, PIKE L M, LIU C T, et al. Correlation of the hardness and vacancy concetration in FeAl[J]. Intermetallics, 1993, 1(2):107–115. DOI:10.1016/0966-9795(93)90028-T |
| [11] | KOGACHI M, HARAGUCHI T. Quenched-in vacancies in B2-structured intermetallic compound FeAl[J]. Materials Science and Engineering:A, 1997, 230(1/2):124–131. |
| [12] | LIU C T, LEE E H, MCKAMEY C G. An environmental effect as the major cause for room-temperature embrittlement in FeAl[J]. Scripta Metallurgica, 1989, 23(6):875–880. DOI:10.1016/0036-9748(89)90263-9 |
| [13] | SUNDARARAJAN V, SAHU B R, KANHERE D G, et al. Cohesive, electronic and magnetic properties of the transition metal aluminides FeAl, CoAl, and NiAl[J]. Journal of Physics:Condensed Matter, 1995, 7(30):6019–6034. DOI:10.1088/0953-8984/7/30/007 |
| [14] | ZOU J, FU C L. Structural, electronic and magnetic properties of 3d transition-metal aluminides with equiatomic composition[J]. Physical Review B, 1995, 51(4):2115–2121. DOI:10.1103/PhysRevB.51.2115 |
| [15] | OUYANG Y F, TONG X F, LI C, et al. Thermodyna-mic and physical properties of FeAl and Fe3Al:An atomistic study by EAM simulation[J]. Physica B:Condened Matter, 2012, 407(23):4530–4536. DOI:10.1016/j.physb.2012.08.025 |
| [16] | MAYER J, ELSÄSSER C, FÄHNLE M. Concentration of atomic defects in B2-FexAl1-x.An ab initio study[J]. Physica Status Solidi:B, 1995, 191(2):283–298. DOI:10.1002/(ISSN)1521-3951 |
| [17] | SCHNEIBEL J H, PIKE L M. A technique for measuring thermal vacancy concentrations in stoichiometric FeAl[J]. Intermetallics, 2004, 12(1):85–90. DOI:10.1016/j.intermet.2003.09.003 |
| [18] | HANC A, KANSY J, DERCZ G, et al. Point defect structure in B2-ordered Fe-Al alloys[J]. Journal of Alloys and Compounds, 2009, 480(1):84–86. DOI:10.1016/j.jallcom.2008.09.177 |
| [19] | ZHAO M, YOSHIMI K, MARUYAMA K., et al. Thermal vacancy behavior analysis through thermal expansion, lattice parameter and elastic modulus measurements of B2-type FeAl[J]. Acta Materialia, 2014, 64:382–390. DOI:10.1016/j.actamat.2013.10.051 |
| [20] | GIALANELLA S, BRUSA R S, DENG W, et al. Defect structures in FeAl B2 alloys[J]. Journal of Alloys and Compounds, 2001, 317/318:485–490. DOI:10.1016/S0925-8388(00)01375-X |
| [21] | WOLFF J, FRANZ M, BROSKA A, et al. Defect types and defect properties in FeAl alloys[J]. Materials Science and Engineering:A, 1997, 239/240:213–219. DOI:10.1016/S0921-5093(97)00584-4 |
| [22] | RIVIÈRE J P, GRILHE J. Restauration de defauts de trempe dans um alliage Fe-Al 40 at.% ordonne[J]. Acta Metallurgica, 1972, 20(11):1275–1280. DOI:10.1016/0001-6160(72)90058-2 |
| [23] | KASS M, BROOKS C R, FALCON D, et al. The formation of defects in Fe-Al alloys:Electrical resistivity and specific heat measurements[J]. Intermetallics, 2002, 10(10):951–966. DOI:10.1016/S0966-9795(02)00115-2 |
| [24] | KERL R, WOLFF J, HEHENKAMP T. Equilibrium vacancy concentrations in FeAl and FeSi investigated with an absolute technique[J]. Intermetallics, 1999, 7(3/4):301–308. |
| [25] | SKOGLUND H, WEDEL M K, KARLSSON B. Thermal vacancy hardening in Fe100-xAlx (28 < x < 40)[J]. Intermetallics, 2007, 15(8):1127–1131. DOI:10.1016/j.intermet.2007.01.008 |
| [26] | KUPKA M. Can vacancies be the main reason of FeAl alloys hardening?[J]. Journal of Alloys and Compounds, 2007, 437(1/2):373–377. |
 2017, Vol. 24
2017, Vol. 24 


